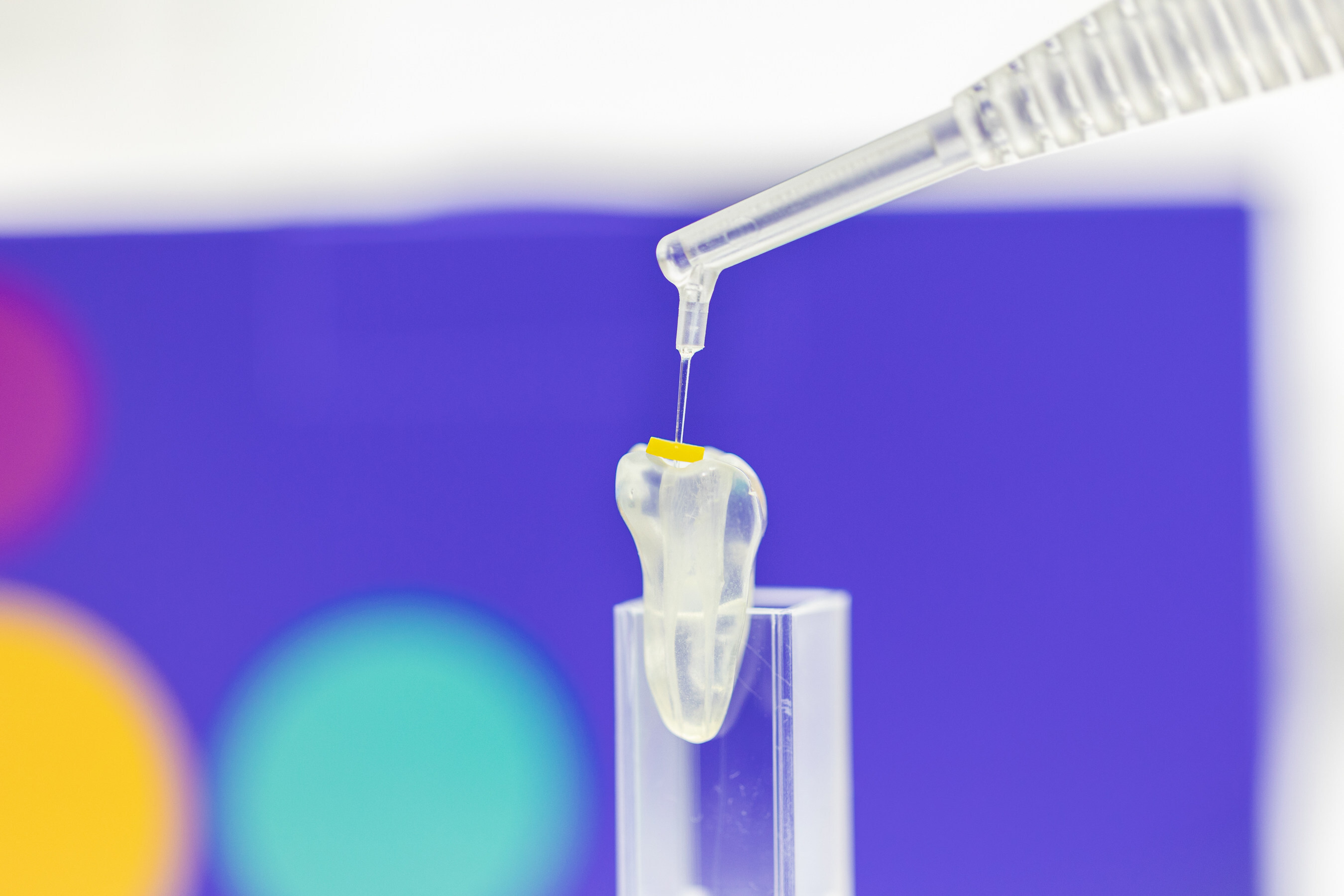
Odne™Clean offers a novel approach to root canal disinfection, simplifying and accelerating the process. It creates a hydro-dynamic cavitation cloud inside the root canal using only saline solution (sodium chloride) as the main irrigation medium. The cavitation jet removes debris inside the canals and tubules and increases the effect of the final disinfection rinse with NaOCl1. Odne™Clean has the potential to reduce the risk of adverse events caused by harsh chemical disinfectants commonly used during root canal disinfection. With its 190 µm tip, the thinnest dental fluid-delivery tip on the market, Odne™Clean supports minimally invasive root canal treatments enabling endodontists and dentists to preserve as much tooth structure as possible. First clinical study results indicate that Odne™Clean results in less post-operative pain compared to other techniques1.
Odne’s Chief Operating Officer and co-founder, Mark Bispinghoff, comments: “With the clearance of Odne™Clean, we now have US market approval for our complete product portfolio for Root Canal Preservation (RPT). We are very proud of our team for going the extra mile and would like to thank our partners for the efficient collaboration in performing the extensive testing the FDA requires.”
In 2023, Odne received FDA-clearance for Odne™Fill, its innovative root canal obturation technique. Odne™Fill is the first light-curing root canal filler that instantly photo-cures upon applying Odne™Cure, a high-precision laser curing light. The three Odne products are a game-changing technology platform for minimally invasive Root Preservation Therapy (RPT) allowing endodontists and dentists to accelerate treatment and increase patient comfort.
“We are thrilled to now integrate Odne™Clean into our Priority Access Program and make it available to our US customer base,” says Andreas Schmocker, CEO and co-founder. “Our goal with the program is to continue to build strong clinical evidence, and, together with our clinicians, we will transform endodontics with a passion for innovation, and scientific and clinical excellence.”
Odne recently initiated its Priority Access Program for innovative Root Preservation Therapy (RPT) at the AAE annual meeting in Los Angeles. The PAP will form the core of Odne’s scientific & clinical community, transforming endodontics. One initiative of the PAP is to perform a clinical case registry, collecting further knowledge on the clinical use of Odne’s devices for root canal treatment. Participating Endodontists get the privilege of being amongst the first users of Odne’s devices and provide valuable use-related and scientific feedback.
1. Internal pre-clinical and clinical data on file
About Odne
Odne AG (formerly Lumendo AG), has its roots in a groundbreaking collaboration between two renowned Swiss institutions: the Swiss Federal Institutes of Technology in Lausanne and Zürich (EPFL & ETH Zürich). Founded in 2018, Odne's journey began by licensing cutting-edge technology assets from these esteemed institutes.
Odne is committed to pioneering the future of endodontics through advanced technology and innovative solutions. Endodontics, the field of dental medicine that deals with root canal treatments, has been marred by complexity and unpredictability. Odne is on a mission to change that.
Root canal treatments have long posed challenges for dentists, with traditionally low success rates ranging from 46% to 91%. With over 60 million annual treatments worldwide, these failures result in significant healthcare costs. Odne's technology platform seeks to address these issues by offering treatment options for both dentists and their patients. Odne's medical device portfolio, launched in the US in April of 2024, tackles problems such as the debridement and obturation of complex root canal morphologies. Recently, Odne Inc, USA, was founded to support the US launch.
Odne is backed by venture capital funds focusing on healthcare and dental. These include Revere Partner (NY), an independent venture fund providing capital for cutting-edge innovations in oral and systemic health, and NV Capital (LIE), a family-owned venture capital boutique led the Series A financing. Alongside the lead investors, other renowned funds, such as Dental Innovation Alliance (DIA), Plug&Play, Hatcher, Züricher Kantonalbank, as well as various family offices and angel investors are supporting Odne's US market launch.
Odne’s technology platform for Root Preservation Therapy (RPT), including: the first hydro-dynamic cavitation device using saline as main debridement medium, and the first all-in-one, light-cured filling material.
Disclaimer: Odne™Fill, Odne™Cure, and Odne™Clean are cleared for use in the U.S.A only.
Pictured: Odne™Clean& – with its 190 µm tip, the thinnest dental fluid-delivery tip on the market.





