New release saves design time and offers more intuitive workflows from CAD to CAM
 exocad, an Align Technology, Inc. company and a leading dental CAD/CAM software provider, today announced the release of DentalCAD 3.1 Rijeka, the next generation of its powerful CAD software for labs and full-service clinics. With more than 45 new and over 85 enhanced features, the new release marks another milestone in exocad’s continuous optimization of workflows for increased productivity.
exocad, an Align Technology, Inc. company and a leading dental CAD/CAM software provider, today announced the release of DentalCAD 3.1 Rijeka, the next generation of its powerful CAD software for labs and full-service clinics. With more than 45 new and over 85 enhanced features, the new release marks another milestone in exocad’s continuous optimization of workflows for increased productivity.
“DentalCAD 3.1 Rijeka is the result of our countless conversations with customers and the dedicated research of our software developers,” said Tillmann Steinbrecher, CEO exocad. “I’m excited about the broad scope of new features and improvements that brings more speed and smoother workflows, from treatment planning to design and manufacturing.”
Easier collaboration between labs and dentists
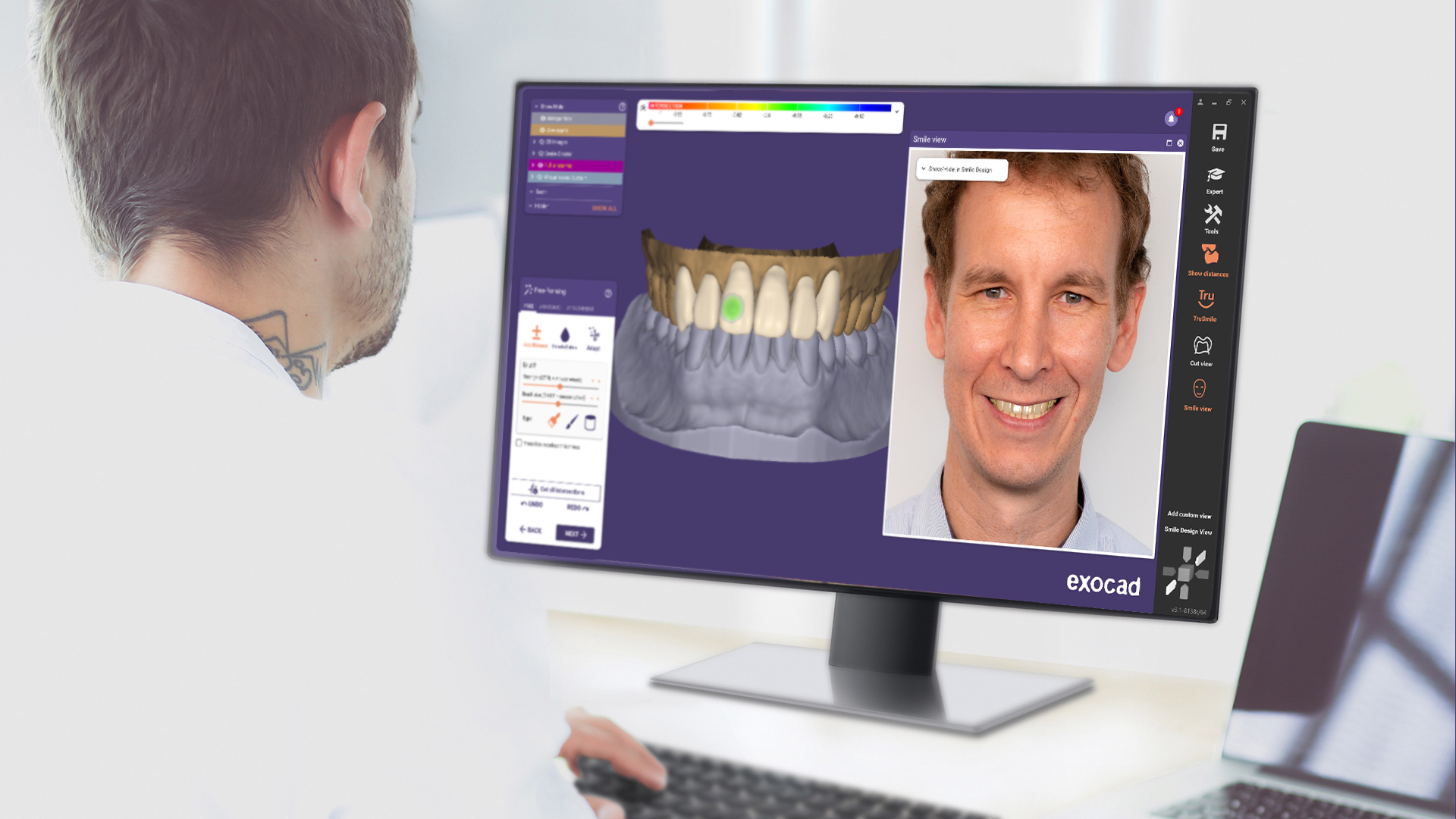
In exocad’s Smile Creator add-on module, many new features await, including more visualization options, such as improved color adjustment for more realistic preview images, and a new slider-based before-and-after view. The new “Smile Design” PDF report provides comprehensive documentation of the esthetic planning process to improve communication between dentists and labs. Additionally, the new “Smile Window” offers instant in-face visualization throughout the CAD workflow. Users see the result of the design within the patient photo in real time.
Get more out of designs
DentalCAD 3.1 Rijeka offers users more efficiency, enabling them to reuse custom tooth setups for multiple indications along the patient case journey. The same shape and setup can be easily used for a mock-up model, a clip-on smile, a temporary restoration, and the final work.
Minimize design time, maximize results
With this new release, exocad speeds up the design of both single-unit restorations and complex, upper and lower restorations. Articulator movements can now be recalculated and visualized on the fly.
DentalCAD 3.1 Rijeka also expands the functionality of Instant Anatomic Morphing, allowing faster design of single-unit restorations with fewer clicks. When a pre-op scan is available, users benefit from an even more highly automated generation of crowns: The previous anatomy copies automatically, making it faster and easier to preserve function and anatomical shape for the patient.
In the Full Denture Module, posterior setups can now be customized as well, providing more control over denture setups. Users can easily expand libraries by saving individual presets, now also in single-arch dentures.
The Model Creator module introduces new “Quick Models” for highly automated model creation, to further save time and minimize the need for user interaction during model design.
Integrate more smoothly with equipment in the lab
exocad’s Virtual Articulator module provides an accurate simulation of more real, physical articulators than any other CAD software*, and in this release, exocad has added even more devices, including the popular Ivoclar and SAM articulators.
The newly modernized exocam, exocad’s integrated CAM solution for milling machines, sets new standards for ease of use. For 3D printing, exoprint now integrates with many additional devices, enabling direct production workflow integration from exocad with more than 40 3D printers, including those of the most popular manufacturers.
Other major highlights of the DentalCAD 3.1 Rijeka release include:
• Improved case documentation with new built-in screenshot management tools for collecting, editing, and tagging screenshots
• Anatomic free-forming features improve ease of use: The affected parts of the design are now visually highlighted, and the user has an overview at a glance of the adaptation parameters to be applied
• The new stock abutment design workflow enables the design of implant-based restorations based on stock abutment libraries
In conjunction with this latest release, exocad launches the new my.exocad portal and introduces end user registration, which will be required to use DentalCAD 3.1 Rijeka.
exocad will offer hands-on demonstrations of the new DentalCAD 3.1 Rijeka release at Insights 2022, the company’s global community event, taking place October 3-4, 2022, in Palma de Mallorca, Spain. The two-day event will include numerous opportunities for attendees to discover more about the latest Rijeka features from dental industry leaders and application specialists. More information is available at exocad.com/insights2022.
DentalCAD 3.1 Rijeka is an essential part of the Align Digital Platform, an integrated suite of proprietary technologies and services delivered as seamless end-to-end solution to customers.
The release is now available in the EU and other select markets. exocad names its releases after current EU “European Capitals of Culture” and selected the Croatian city of Rijeka for this year’s release.
Additional information is available at exocad.com/our-products/dentalcad-rijeka
*Data on file









 exocad, an Align Technology, Inc. company and a leading dental CAD/CAM software provider, today announced the release of DentalCAD 3.1 Rijeka, the next generation of its powerful CAD software for labs and full-service clinics. With more than 45 new and over 85 enhanced features, the new release marks another milestone in exocad’s continuous optimization of workflows for increased productivity.
exocad, an Align Technology, Inc. company and a leading dental CAD/CAM software provider, today announced the release of DentalCAD 3.1 Rijeka, the next generation of its powerful CAD software for labs and full-service clinics. With more than 45 new and over 85 enhanced features, the new release marks another milestone in exocad’s continuous optimization of workflows for increased productivity.
 Zest Dental Solutions, the only manufacturer of the Zest LOCATOR® Family of Abutment Systems, including the LOCATOR® Overdenture Implant System, announces a significant legal victory which solidifies and reinforces the legitimacy of its LOCATOR® product line.
Zest Dental Solutions, the only manufacturer of the Zest LOCATOR® Family of Abutment Systems, including the LOCATOR® Overdenture Implant System, announces a significant legal victory which solidifies and reinforces the legitimacy of its LOCATOR® product line.




