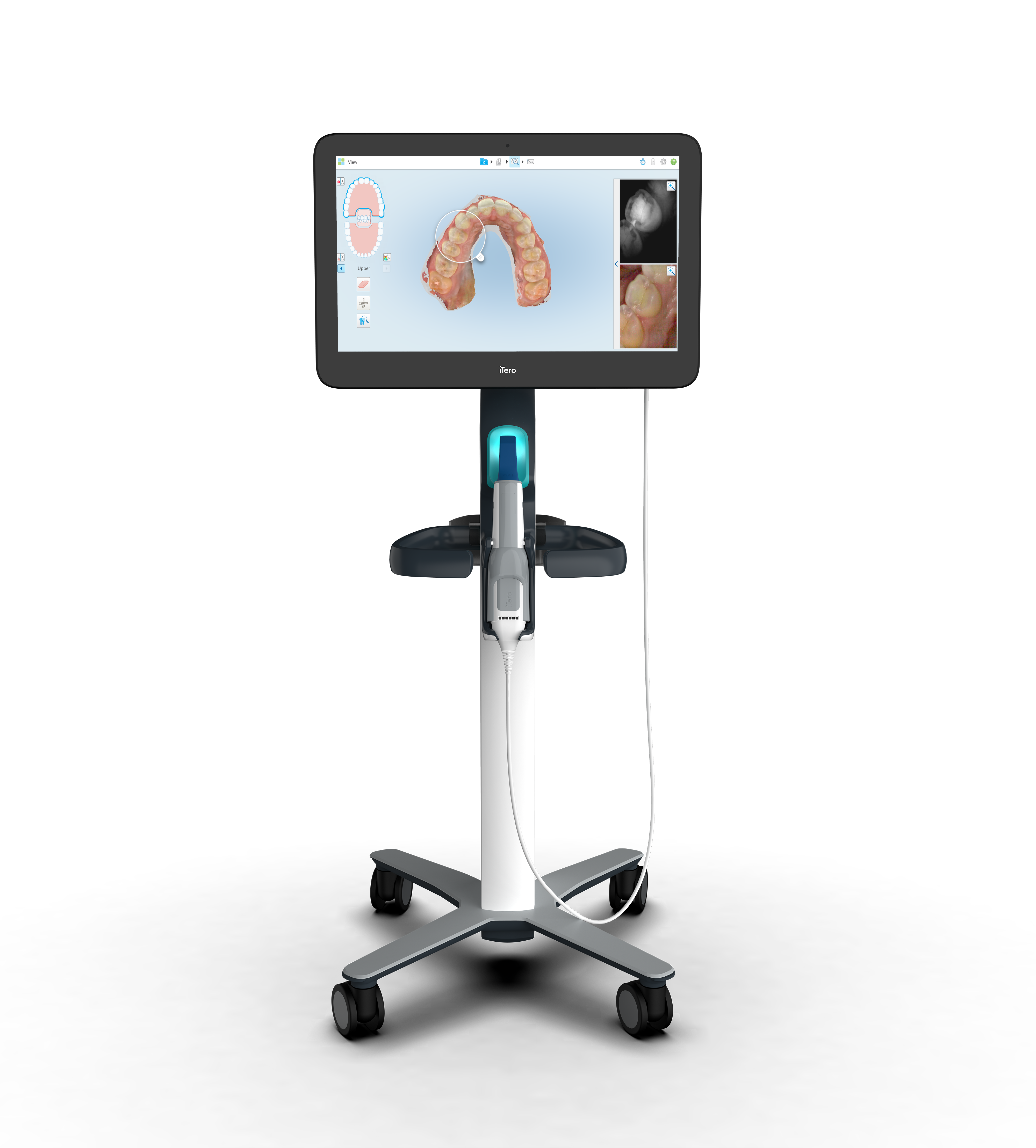
 Align Technology, Inc. (“Align”) (Nasdaq: ALGN) a leading global medical device company that designs, manufactures, and sells the Invisalign system of clear aligners, iTero intraoral scanners, and exocad CAD/CAM software for digital orthodontics and restorative dentistry, today announced the new iTero Workflow 2.0 software release with advanced features that provide enhanced intraoral image sharpness for clearer hard and soft tissue details to aid in treatment diagnosis, while also driving practice efficiency, patient engagement, and a more seamless end-to-end digital treatment experience for doctors and their patients.
Align Technology, Inc. (“Align”) (Nasdaq: ALGN) a leading global medical device company that designs, manufactures, and sells the Invisalign system of clear aligners, iTero intraoral scanners, and exocad CAD/CAM software for digital orthodontics and restorative dentistry, today announced the new iTero Workflow 2.0 software release with advanced features that provide enhanced intraoral image sharpness for clearer hard and soft tissue details to aid in treatment diagnosis, while also driving practice efficiency, patient engagement, and a more seamless end-to-end digital treatment experience for doctors and their patients.
“Align’s commitment to innovation in digital orthodontics and restorative dentistry reflects our $250 million annual investment in technology to develop products and services that provide doctors and their patients with a great treatment outcome and seamless experience through the Align digital platform,” said Yuval Shaked, Align SVP and MD of the iTero systems and services business. “Our new iTero Workflow 2.0 software features were developed to simplify and streamline a doctor’s daily routine and increase practice efficiency. From a faster, all-in-one scan with enhanced visualization capabilities to improved patient communication, including the ability to capture, annotate and then share Invisalign simulations or restorative treatment plans digitally, these new features provide doctors with the ability for better clinical diagnosis and help patients better understand their oral health conditions and the proposed treatment options.”
The newly released iTero Workflow 2.0 software features include:
Faster scanning: Enables a faster and smoother all-in-one scan for maximum efficiency with 20% less waiting for processing time on the iTero Element Plus Series scanners1 and 50% faster movement and 25% faster rotation during scanning2 for efficient daily use and ease of learning on all iTero Element scanners.
Improved visualization: The integrated 3D intraoral camera included in the iTero Element 5D Plus imaging system provides enhanced sharpness and improved image quality powered by advanced AI capabilities to deliver clearer soft and hard tissue details to support diagnosis3. In addition, the enhanced capabilities allow clinics to efficiently use intraoral scan images in place of traditional intraoral photos as they can capture multiple intraoral images at different angles automatically with one scan.
Next level patient communication tools: The new Snapshot tool and iTero Scan Report provide doctors and their staff with the ability to capture information such as Invisalign Outcome Simulator projections and share it digitally with their patients, allowing patients to make more confident decisions – in the dental chair or at home – which may lead to higher treatment acceptance.
“I am already experiencing meaningful differences with the new iTero software features and have seen significantly reduced scan times,” said Dr. Olivier Boujenah, a dentist in France who participated in the limited market release. “The intraoral images are much sharper, and my patients are impressed by the details and clarity, which leads them to ask more questions about treatment options. Being able to send digital files with patients when they leave the office through the iTero Scan Report keeps our conversation going even after they are home and I already see this capability helping with patient acceptance.”
iTero Workflow 2.0 software features are being rolled out regionally and are expected to be available in all markets where the iTero Element Plus imaging systems are available by the end of Q2’21. Software features will vary by scanner. Discover more at https://bit.ly/2ROw0fD.
New iTero Element 5D imaging system auto-upload functionality
The company also announced that new iTero Element 5D imaging system auto-upload of intraoral photos for Invisalign case submissions will be available in the third quarter of 2021. This new functionality will eliminate steps and streamline Invisalign case submissions with intraoral color scan images that can be used in place of traditional intraoral photos.
The iTero Element 5D imaging system wands are designed with advanced technology, including an intraoral camera, that enables the doctor to scan, capture and auto convert the scan into 2D color photos. This enhanced capability provides doctors the option to automatically populate the five required images in the prescription form in the Invisalign Doctor Site (IDS) with 2D color scan photos. Currently, the Invisalign prescription form requires at least five intraoral photos upon submission, taken with Invisalign Photo Uploader (IPU) or a digital camera. This new auto upload functionality will be available in all markets where the iTero Element 5D imaging systems are sold.
“I am excited to hear about the new iTero Element 5D auto-upload feature,” said Dr. Heather Stone Hopkins, an orthodontist in Lexington, South Carolina and an Align North America faculty member. “Digital technology such as the iTero scanner has been a game changer for my practice. In addition to eliminating goopy PVS impressions, the iTero Element 5D also simultaneously records 3D, intraoral color and NIRI images — eliminating the need for multiple devices. With this new iTero Element 5D feature, intraoral color scan images can be used in place of the required intraoral photos for Invisalign case submissions, and without the discomfort of cheek retractors for a better patient experience. Automatic intraoral image capture and auto-upload to the Invisalign Doctor Site is an enhancement that will drastically improve our workflow, saving my team members and patients invaluable time for our Invisalign case submissions.”
Data on file, as of December 22, 2020.
Compared with previous software versions. Data on file at Align Technology, as of March 29, 2021.
Only available on iTero Element 5D Plus imaging systems (full and Lite software configurations).
*iTero Element 5D Imaging systems include iTero Element 5D and iTero Element 5D Plus series scanners.
About Align Technology, Inc.
Align Technology designs, manufactures and offers the Invisalign system, the most advanced clear aligner system in the world, iTero intraoral scanners and services, and exocad CAD/CAM software. These technology building blocks enable enhanced digital orthodontic and restorative workflows to improve patient outcomes and practice efficiencies for over 200 thousand doctor customers and is key to accessing Align’s 500 million consumer market opportunity worldwide. Align has helped doctors treat over 10.2 million patients with the Invisalign system and is driving the evolution in digital dentistry through the Align Digital Platform, our integrated suite of unique, proprietary technologies and services delivered as a seamless, end-to-end solution for patients and consumers, orthodontists and GP dentists, and lab/partners. Visit www.aligntech.com for more information.
For additional information about the Invisalign system or to find an Invisalign doctor in your area, please visit www.invisalign.com. For additional information about iTero digital scanning system, please visit www.itero.com. For additional information about exocad dental CAD/CAM offerings and a list of exocad reseller partners, please visit https://www.exocad.com.
Forward-Looking Statements
This news release contains forward-looking statements, including quotations and expectations regarding product and services development and spending, the capabilities of new features and functionalities, and our expectations for our new products, features, and accessories and their availability. Forward-looking statements contained in this news release relating to expectations about future events or results are based upon information available to Align as of the date hereof. Readers are cautioned that these forward-looking statements are only predictions and are subject to risks, uncertainties, and assumptions that are difficult to predict. As a result, actual results may differ materially and adversely from those expressed in any forward-looking statement.
Factors that might cause such a difference include, but are not limited to:
the impact of the COVID-19 pandemic on the health and safety of our employees, customers, patients, and our suppliers, as well as the physical and economic impacts of the various recommendations, orders, and protocols issued by local and national governmental agencies in light of continual evolution of the pandemic, including any periodic reimplementation of preventative measures in various global locations;
unexpected or rapid changes in the growth or decline of our domestic and/or international markets;
increasing competition from existing and new competitors;
rapidly evolving and groundbreaking advances that fundamentally alter the dental industry or the way new and existing customers market and
provide products and services to consumers;
the ability to protect our intellectual property rights;
continued compliance with regulatory requirements;
declines in, or the slowing of the growth of, sales of our intra-oral scanners domestically and/or internationally and the impact either would have on the adoption of Invisalign products;
the willingness and ability of our customers to maintain and/or increase product utilization in sufficient numbers;
the possibility that the development and release of new products or enhancements to existing products do not proceed in accordance with the anticipated timeline or may themselves contain bugs or errors requiring remediation and that the market for the sale of these new or enhanced products may not develop as expected;
the risks relating to our ability to sustain or increase profitability or revenue growth in future periods (or minimize declines) while controlling expenses; and the loss of key personnel or work stoppages.
The foregoing and other risks are detailed from time to time in our periodic reports filed with the Securities and Exchange Commission, including, but not limited to, our Annual Report on Form 10-K for the year ended December 31, 2020, which was filed with the Securities and Exchange Commission (SEC) on February 26, 2021. Align undertakes no obligation to revise or update publicly any forward-looking statements for any reason.





 Benco Dental, the nation's largest independent distributor of oral healthcare technology and supplies, has finalized a definitive agreement to purchase the assets of ADI Mobile Health.
Benco Dental, the nation's largest independent distributor of oral healthcare technology and supplies, has finalized a definitive agreement to purchase the assets of ADI Mobile Health.
 Align Technology, Inc. (“Align”) (Nasdaq: ALGN) a leading global medical device company that designs, manufactures, and sells the Invisalign system of clear aligners, iTero intraoral scanners, and exocad CAD/CAM software for digital orthodontics and restorative dentistry, today announced the new iTero Workflow 2.0 software release with advanced features that provide enhanced intraoral image sharpness for clearer hard and soft tissue details to aid in treatment diagnosis, while also driving practice efficiency, patient engagement, and a more seamless end-to-end digital treatment experience for doctors and their patients.
Align Technology, Inc. (“Align”) (Nasdaq: ALGN) a leading global medical device company that designs, manufactures, and sells the Invisalign system of clear aligners, iTero intraoral scanners, and exocad CAD/CAM software for digital orthodontics and restorative dentistry, today announced the new iTero Workflow 2.0 software release with advanced features that provide enhanced intraoral image sharpness for clearer hard and soft tissue details to aid in treatment diagnosis, while also driving practice efficiency, patient engagement, and a more seamless end-to-end digital treatment experience for doctors and their patients.

 Glidewell has launched the BruxZir® Zirconia Shade Guide, the world's first shade-taking system designed specifically for BruxZir Zirconia dental restorations.
Glidewell has launched the BruxZir® Zirconia Shade Guide, the world's first shade-taking system designed specifically for BruxZir Zirconia dental restorations. 
