Today, the American Dental Hygienists’ Association installed Michele Braerman, RDH, BSDH, of Fallston, Maryland, as the organization’s 2018-19 president, along with ADHA’s new slate of officers for the upcoming year. “With a rapidly changing oral care environment, there is no better time for ADHA to be united and to foster growth and change for our profession,” said Braerman. “I am privileged to collaborate with this great leadership team to move the organization and profession forward.”
A member of ADHA since her graduation in 1992, Braerman has served the Association in a wide range of positions, including most recently as president-elect of ADHA, District III trustee, a member of the executive and finance committee, a 2-year board coach, chair of Council on Public Relations, a MDHA delegate and reference committee chair. She also served her constituent and component in numerous leadership roles which include: secretary, vice president and president. MDHA honored her with the Symbol of Excellence Award in 2010. Braerman holds a BS degree in dental hygiene from the University of Maryland School of Dentistry in Baltimore. She currently works as a clinician in a private practice and previously as clinical faculty at the Community College of Baltimore County. Braerman succeeds Tammy Filipiak, RDH, MS, of Mosinee, Wisconsin, as president. Filipiak will remain on the Board of Trustees serving both as ADHA immediate past-president as well as the 2018-19 chair of the ADHA Institute for Oral Health.
Additional ADHA officers newly installed at the ADHA’s House of Delegates Meeting include: Matt Crespin, RDH, MPH, of Milwaukee, Wisconsin, as president-elect; Lisa Moravec, RDH, MS, of Scottsbluff, Nebraska, as vice president; and Donnella Miller, RDH, BS, MPS, of Des Moines, Iowa, as treasurer.
The newly installed district trustees for 2018-2019 are: Becky Smith, CRDH, EdD, of Miami, District IV (Fla., Ga., N.C., S.C.) Erin Haley-Hitz, RDH, MS, PhRDH, Om of Lincoln, Nebraska, District VIII (Ill., Iowa, Kan., Mo., Neb.); Crystal Spring, RDH, BS, of Bozeman, Montana, District X (Colo. Mont., Utah, Wyo.).
District trustees returning in 2018-2019 include: Peter Gangi, RDH, BS Ed, of Methuen, Massachusetts, District I (Maine, Mass., N.H., R.I., Vt.); Donna Lee Hickey, RDH, of Bethpage, New York, District II (Conn., N.J., N.Y., Pa.); Dawn Ann Dean, RDH, MSDH, of Wheeling, West Virginia, District III (Del., District of Columbia., Md., Va., W.Va.); Sharlee Burch, BSDH, MPH, EdD, of Lexington, Kentucky, District V (Ind., Ky., Mich., Ohio); Danielle Victoriano, RDH, BS, MHS, of Slidell, Louisiana, District VI (Ala., Ark., La., Miss., Tenn.); Rachelle Gustafson, RDH, of Thompson, North Dakota, District VII (Minn., N.D., S.D., Wis.); Cynthia Baty, RDH, BS, of Tulsa, Oklahoma, District IX (N.M., Okla., Texas); Trinity Cleveland, RDH, of Chandler, Arizona, District XI (Ariz., Calif.); Annette Lincicome, RDH, MSDH, of Las Vegas, Nevada, District XII (Alaska, Hawaii, Idaho, Nev., Ore., Wash.)




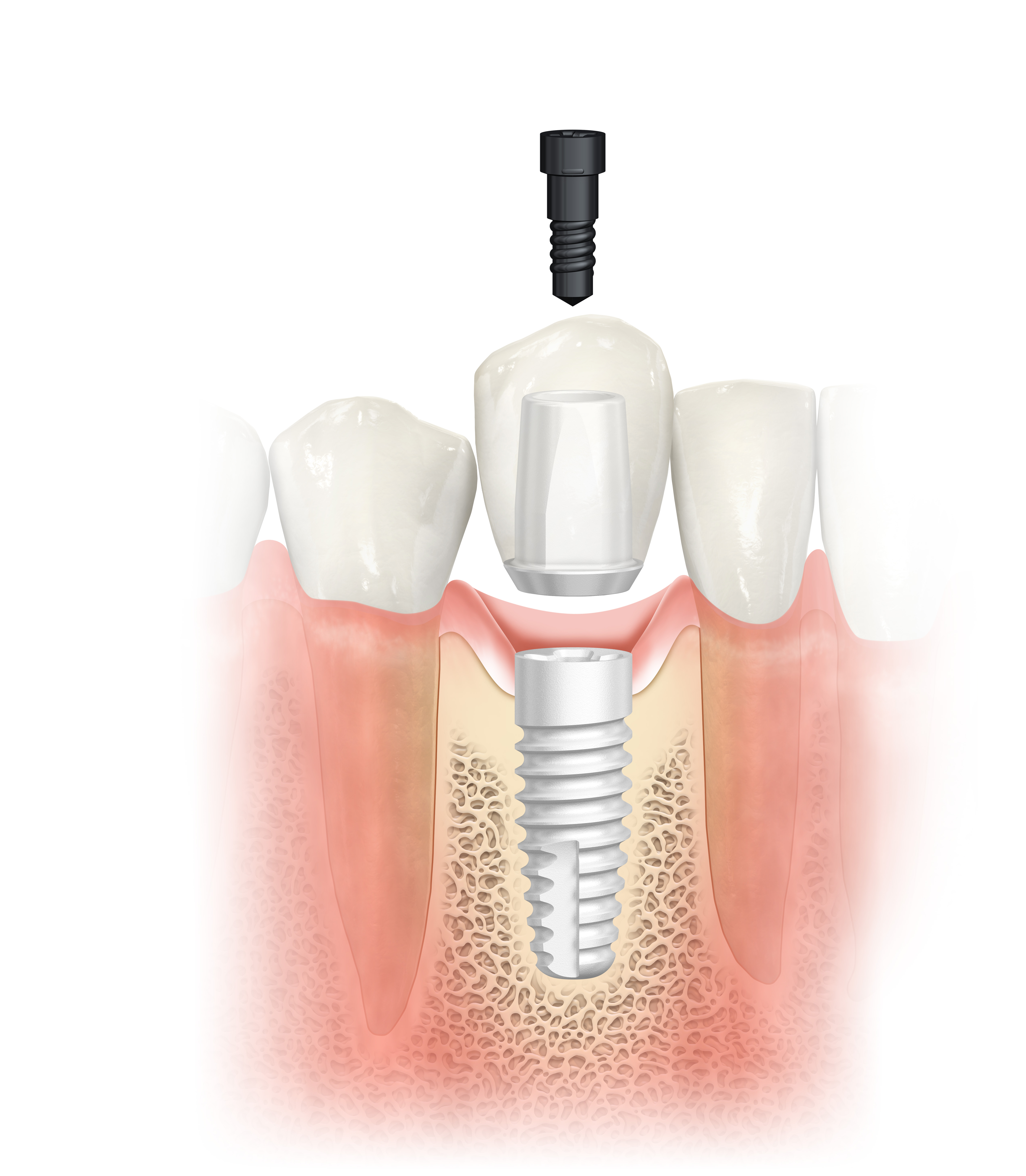 Dental professionals can now add a splash of life to their treatment portfolio with NobelPearl ceramic implants launched by Nobel Biocare during EuroPerio9, June 20-23, in Amsterdam. Recent trends in the dental implant market indicate that patients increasingly prefer metal-free solutions for the look and feel of natural teeth. With NobelPearl, dental professionals can now give them the esthetics they desire, as the two-piece ceramic implant with a cement-free internal connection supports a natural soft-tissue appearance.
Dental professionals can now add a splash of life to their treatment portfolio with NobelPearl ceramic implants launched by Nobel Biocare during EuroPerio9, June 20-23, in Amsterdam. Recent trends in the dental implant market indicate that patients increasingly prefer metal-free solutions for the look and feel of natural teeth. With NobelPearl, dental professionals can now give them the esthetics they desire, as the two-piece ceramic implant with a cement-free internal connection supports a natural soft-tissue appearance.


 At EuroPerio9 in Amsterdam, The Netherlands, Nobel Biocare is announcing a global exclusive partnership agreement with GalvoSurge Dental AG, a Swiss-based manufacturer of dental devices. The two companies intend to bring to market a new and innovative cleaning system for long-term implant maintenance on all major implant brands.
At EuroPerio9 in Amsterdam, The Netherlands, Nobel Biocare is announcing a global exclusive partnership agreement with GalvoSurge Dental AG, a Swiss-based manufacturer of dental devices. The two companies intend to bring to market a new and innovative cleaning system for long-term implant maintenance on all major implant brands.



