With a digital base, historical product strength and signature workflows supported by ongoing education and training, Dentsply Sirona is launching DS Implants, harmonizing successful brands like Simplant, OSSIX, Atlantis and MIS. Three completely connected, seamless signature workflows will take full advantage of digital dentistry for excellent outcomes and patient satisfaction. DS Implants presents DS PrimeTaper, a self-tapping implant with a tapered design that can be inserted safely and easily with a unique double thread, enabling long-term bone stability.
 Today, Dentsply Sirona introduces a comprehensive restage of its Implants business, including three signature workflows to provide dental professionals with a completely new way of practicing implantology and solutions from scan to crown that outperform expectations in efficiency, accuracy, esthetics, longevity and simplicity.
Today, Dentsply Sirona introduces a comprehensive restage of its Implants business, including three signature workflows to provide dental professionals with a completely new way of practicing implantology and solutions from scan to crown that outperform expectations in efficiency, accuracy, esthetics, longevity and simplicity.
● The Single Tooth Signature Workflow enables customers to do more and better, faster, simpler, freeing up time to see more patients.
●The Partial Multiple Tooth Replacement Signature Workflow solves a signature patient case with three posterior teeth missing to be restored with a three-unit bridge on two implants for more confidence for the clinician and more comfort for the patient.
●The Full Arch Signature Workflow addresses a signature patient case with an edentulous maxilla. The workflow offers a restoration with a fix bridge on four implants with immediate temporization.
With a complete ecoystem of digital solutions and products – including well-known brands like Simplant, OSSIX, Axeos, Primescan, Atlantis and MIS – Dentsply Sirona is streamlining implant treatments for practice growth and consistent patient satisfaction.
Comfort with new products based on well-known procedures
Additionally, Dentsply Sirona Implants presents DS PrimeTaper, a self-tapping implant with a tapered design that can be inserted safely and easily. This enables long-term bone stability. The double thread allows the implant to be inserted quickly and safely. A simple drilling protocol, with only a few different drills, supports the workflow.
Dentsply Sirona worked closely with the global implant key opinion leader community, and they have already started gaining experience with the product. Their reactions have been very positive:
Dr. Dan Butterman, dentist from Centennial, Colorado (USA), emphasizes the importance of a seamless workflow. “DS PrimeTaper represents the next step in implant dentistry. The digital-driven workflow results in higher accuracy, repeatability of processes, and time savings.” Butterman adds, "My patients have better things to do than spending time in my office. Thanks to clearly defined processes and digital solutions, I can offer safer and faster treatment options which my patients appreciate. That's what efficiency is all about!"
Dr. Martin Wanendeya, dentist from the United Kingdom, notes "DS PrimeTaper as a part of an integrated concept is different from what we knew before. In addition to reducing chair time and simplifying collaboration with my laboratory, Primescan for Atlantis suprastructures ensures aesthetic predictability and a perfect fit.”
Seamless concept for transforming digital implant dentistry
“Dentsply Sirona is about intelligent concepts that work intuitively and improve the predictability of treatment,” said Dr. Terri Dolan, Chief Clinical Officer at Dentsply Sirona. “Our goal is to enable clinicians, including both specialists and general practitioners, to focus on what they do best – creating healthy and beautiful smiles. We are confident that we are building a winning future in the area of implant solutions focused on the needs of our implant customers and their patients. Our connected and seamless concept for transforming digital implant dentistry is a first, essential step in this direction. Our vision for digital implant dentistry will be supported by a global clinical education program focused on end-to-end digital implant workflow solutions to achieve clinic, technical and practice excellence. The extensive curriculum is designed to build clinicians’ digital implant skills according to their level of experience and personal learning preferences. These include online education and personalized learning, as well as more than 50 state-of-the art Dentsply Sirona Academy education centers around the world.”
Start of clinical and registry study at Dentsply Sirona World
In cooperation with the McGuire Institute (iMc), two levels of evidence will be covered: The basis is provided by a prospective, multicenter, clinical study evaluating the complete digital workflow including 10 private practice investigators and their referrals for a five-year follow-up.
In addition, an ambitious registry study has been initiated aiming to involve more than 500 clinicians, placing over 2.500 implants for long-term follow-up. The beauty of a registry study is to obtain long-term clinical data without being prescriptive or having a specific endpoint. Therefore, it’s a powerful tool to create scientific evidence and market insights from the daily use of the product by the clinician. Both the clinical trial and the registry will collect “Real World Evidence” which is now recognized by the FDA as being the standard in collecting data and is meaningful to clinicians and patients alike.
Due to different approval and registration times, not all technologies and products are immediately available in all countries





 exocad GmbH (exocad), an Align Technology, Inc. company and a leading dental CAD software provider, detailed the company's achievements over the past year and recent productivity-boosting software innovations at its International Dental Show (IDS) press event. Under the new slogan "Imagine the CADabilities," exocad executives highlighted new product launches, recently formed strategic partnerships and company milestones.
exocad GmbH (exocad), an Align Technology, Inc. company and a leading dental CAD software provider, detailed the company's achievements over the past year and recent productivity-boosting software innovations at its International Dental Show (IDS) press event. Under the new slogan "Imagine the CADabilities," exocad executives highlighted new product launches, recently formed strategic partnerships and company milestones.


 Globally, 1 in 700 babies are born with a cleft lip and/or palate. Clefts can have devastating effects on the quality of life of a child, both physically by compromising the ability to communicate and to eat properly and from social stigma resulting in feelings of anxiety and a lack of self-confidence. A child with a cleft requires more than just surgery. Essential cleft care includes nutrition programs, dental and orthodontic care, speech therapy, and social and emotional support.
Globally, 1 in 700 babies are born with a cleft lip and/or palate. Clefts can have devastating effects on the quality of life of a child, both physically by compromising the ability to communicate and to eat properly and from social stigma resulting in feelings of anxiety and a lack of self-confidence. A child with a cleft requires more than just surgery. Essential cleft care includes nutrition programs, dental and orthodontic care, speech therapy, and social and emotional support.

 Today, Dentsply Sirona introduces a comprehensive restage of its Implants business, including three signature workflows to provide dental professionals with a completely new way of practicing implantology and solutions from scan to crown that outperform expectations in efficiency, accuracy, esthetics, longevity and simplicity.
Today, Dentsply Sirona introduces a comprehensive restage of its Implants business, including three signature workflows to provide dental professionals with a completely new way of practicing implantology and solutions from scan to crown that outperform expectations in efficiency, accuracy, esthetics, longevity and simplicity.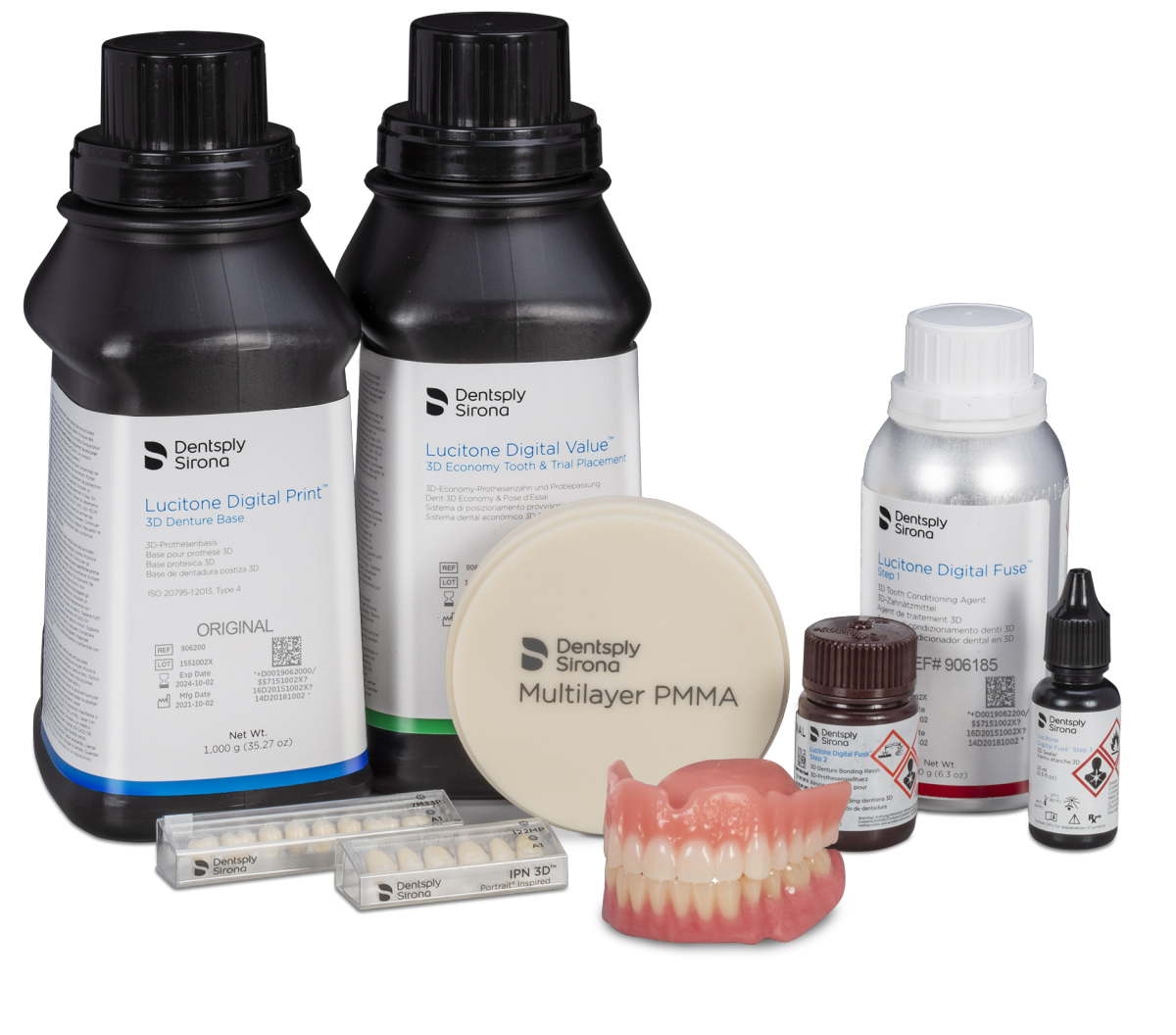 Dentsply Sirona announced today four expansions of the Lucitone Digital Print Denture System, adding momentum to the digital transformation of the dental laboratory industry. Three of these expansions are for the digital production of custom tooth arches and segments, including the addition of Lucitone Digital Value 3D Economy Tooth and Trial Placement resin; DS Multilayer PMMA Discs for milling premium denture teeth; and the planned 2022 release of Lucitone Digital IPN, a printed premium denture tooth material. Later in 2021, planned printer validations for the Lucitone Digital Print Denture System will include Asiga Max UV and Pro 4K and SprintRay Pro 95 and Pro 55 series printers. Lucitone Digital Value and DS Multilayer PMMA denture tooth workflows are validated and immediately available for Carbon M-Series printers.
Dentsply Sirona announced today four expansions of the Lucitone Digital Print Denture System, adding momentum to the digital transformation of the dental laboratory industry. Three of these expansions are for the digital production of custom tooth arches and segments, including the addition of Lucitone Digital Value 3D Economy Tooth and Trial Placement resin; DS Multilayer PMMA Discs for milling premium denture teeth; and the planned 2022 release of Lucitone Digital IPN, a printed premium denture tooth material. Later in 2021, planned printer validations for the Lucitone Digital Print Denture System will include Asiga Max UV and Pro 4K and SprintRay Pro 95 and Pro 55 series printers. Lucitone Digital Value and DS Multilayer PMMA denture tooth workflows are validated and immediately available for Carbon M-Series printers.




