Contaminants on Titanium and Ceramic Implants
Manufacturing and packaging deficits may contribute to the development of peri-implantitis
Scott D. Ganz, DMD | Dirk U. Duddeck, DDS Gregori M. Kurtzman, DDS | Kenneth S. Serota, DDS, MMSc
Dental implants have become standard treatment for the replacement of missing teeth, from single units to fully edentulous arches, and they have demonstrated a very high level of success for decades. Modern dental implants are designed to replace the root of the missing tooth and serve as the foundation for the desired restoration. Whether implants are titanium or ceramic (ie, zirconia), they "bond" to the bone through a process called osseointegration in which the bone attaches to the implant surface. Although dental implants have become a mainstream treatment option, the profession is witnessing an increase in the reported incidence of peri-implantitis and accompanying peri-implant bone loss. These reports relate to bone loss at the interface of the implant surface, which is calibrated as bone-to-implant contact. Fortunately, it is commonly accepted that the incidence of peri-implantitis is not actually increasing; the identification of it is.1
Etiology of Peri-Implant Disease
Peri-implant disease is classified into peri-implant mucositis and peri-implantitis. Peri-implant mucositis is characterized by inflammation that is found only in the soft tissues adjacent to the implant (ie, gingival cuff) with no evidence of bone loss.2 Generally, peri-implant mucositis is a precursor to peri-implantitis, and when identified early enough, it may be successfully treated to prevent progression to peri-implantitis. Progression occurs when the gingival inflammation spreads to the underlying bone and leads to deterioration of the osseous support around the implant. Peri-implantitis is characterized by inflammation of the soft and hard tissues adjacent to implants that has resulted in progressive bone loss.2,3 When not identified and treated early enough, this loss of bone can eventually lead to de-osseointegration of the implant. The treatment of peri-implantitis typically requires surgical intervention to stop further progression and repair the lost hard tissues via osseous grafting. Therefore, the timely identification of peri-implant mucositis and early intervention to prevent its progression to the hard tissues is key to the clinical success of implants.
Peri-implant disease may occur early in the life of an implant or many years following its restoration. Depending on the causative factor, the inflammatory process can progress slowly or in an accelerated manner. Various factors that can lead to peri-implant disease include issues related to the patient's immune system, uncontrolled diabetes, poor at-home hygiene care, a lack of keratinized tissue surrounding the implant, smoking, occlusal interferences, periodontal disease, and more. Dental implants require routine monitoring as part of a comprehensive periodontal evaluation.4 In the literature, the reported incidence of peri-implant mucositis and peri-implantitis ranges from 46% to 63% and 19% to 23%, respectively.5-8 Because peri-implant disease affects a significant number of implant patients, it is necessary to understand its diagnosis and the risk factors that can be modified to reduce the potential for its occurrence or limit its progression. Implant-supported restorations, as with natural teeth, require regular home care to maintain the soft tissue, which acts as a barrier to the deeper progression of inflammatory factors.
In recent years, there has been a greater focus on peri-implantitis due to the increase in its reported incidence. This increase is related to more frequent identification as practitioners increasingly have come to understand the signs and symptoms associated with the disease.4,9However, the clinical and histological conditions that drive the conversion from gingival inflammation to peri-implantitis are still not well understood.10 Histologically, at the clinical level, sites with peri-implantitis often have larger inflammatory lesions than sites with periodontitis around natural teeth.11,12 The evidence suggests that progressive crestal bone loss around implants in the absence of clinical signs of soft-tissue inflammation is rare.10
Factors Affecting Peri-Implant Disease
Evidence has demonstrated an increased risk of the development of peri-implantitis in patients who have a history of chronic periodontitis, who have poor plaque control skills, and/or who fail to maintain regular maintenance care following the completion of implant treatment. In addition, the literature reports that smokers have a higher incidence of peri-implantitis (72.7%) when compared with nonsmokers (27.3%) and that a higher richness of microbiota is identified in patients with peri-implantitis who are smokers.13,14 Research shows that patients with periodontitis demonstrated a 50% chance of having peri-implantitis.15,16Those who have oral inflammatory changes associated with their remaining natural teeth are at a much greater risk of acquiring peri-implantitis. Moreover, the oral biofilm in periodontally involved patients increases their immunological sensitivity to further insult from particles that are present around or on the implant surface. It has also been reported that patients who are allergic to penicillin experience double the normal dental implant failure rate, which may also be related to receiving alternative antibiotic therapy.17 Although systemic diseases, such as arterial hypertension, diabetes mellitus, osteoporosis, and cardiovascular diseases, have not been shown to have a statistically significant influence on the incidence of peri-implantitis, appropriate management of the risk factors associated with peri-implant disease, including improving home care, treating periodontal issues, and addressing systemic health issues (eg, diabetes), can aid in decreasing the incidence of peri-implantitis and its severity.7
Sterile Implants May Be Contaminated
An understated risk factor to the development of peri-implant disease that has become a significant focus in the dental implant industry is one that arises during the manufacturing and packaging of dental implants. The level of quality control maintained during the manufacture of dental implants and the sterility maintained during their packaging are largely unknown and underestimated factors that can affect the short- and long-term condition of implants once inserted intraorally. How a dental implant is manufactured and packaged may significantly influence how its surface interacts with the surrounding bone in the early and even later stages of osseointegration.18
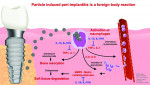
The manufacturing process needs to be meticulous in all phases to ensure that the end product is not only sterile but also free of surface contaminants that can trigger immunological reactions that are technically avoidable. Even if an implant is sterile when removed from the manufacturer's packing, thin-film contaminants, which are organic and/or non-organic particles directly related to the complex and elaborate manufacturing process, may be present on its surface.19 Any particles of material, whether they are metallic or carbonaceous organic particles left on the implant's surface following manufacturing or plastic particles resulting from the packaging, can initiate an immune response in a patient via a foreign body reaction (Figure 1). During that foreign body response, tumor necrosis factor alpha (TNF-α) promotes osteoclastic activity that leads to bone loss at the osseous interface with the implant.20
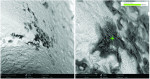
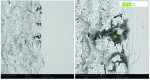
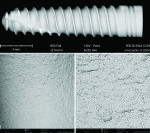
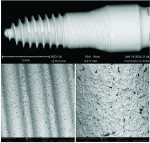
Dental implant quality assessment studies conducted by the CleanImplant Foundation in collaboration with Charité University Medicine in Berlin, Germany, and the Sahlgrenska Academy in Gothenburg, Sweden, have utilized scanning electron microscopy (SEM) to identify impurities on new sterile-packaged titanium and ceramic dental implants. The findings regarding particulate contamination that have been reported are specific to the processes used during the manufacture and packaging of those implants.19,21 According to the reported data, one out of three implant systems analyzed demonstrated significant amounts of factory-related impurities on the implants' surfaces when removed from the packaging.22 Those contaminants included organic particles from the manufacturing process; metallic particles that contained, inter alia, iron-chromium compounds, nickel, tungsten, copper, and tin from the milling or surface treatment process; and plastic from handling and packaging. The areas on an implant that may present with contamination include the shoulder area at the implant's platform (Figure 2) and along the implant's threads (Figure 3).18
When viewed under SEM, some of the implants examined demonstrated not only isolated spots of impurities but also larger areas that were either insufficiently cleaned during the production process or contaminated without being noticed during packaging. Under high magnification in the SEM imaging, organic carbonaceous particles appeared as black spots; however, the SEM images in both low magnification (500×) and high magnification (2,500×) also revealed thermoplastic materials, synthetic polymers, and polysiloxanes on the sterile implant surfaces. This has been identified in both titanium implants (Figure 4 through Figure 7) and ceramic implants (Figure 8) from various manufacturers. Following removal from the manufacturer's packaging, some of the ceramic implants demonstrated significant organic debris related to the packaging material when analyzed by SEM (Figure 9).20 However, implants that were properly treated following manufacturing and appropriately packaged demonstrated surfaces that were devoid of organic, metallic, and plastic particles when examined under SEM (Figure 10 and Figure 11).
Although all implant surface contaminants pose risk, organic carbonaceous particles that detach from the implant surface during the insertion process have been associated with peri-implant bone loss and peri-implantitis.21 Macrophages take up the particles by phagocytosis and subsequently release a storm of pro-inflammatory cytokines such as TNF-α, and interleukins (eg, IL-1b, IL-6). These cytokines stimulate the differentiation of osteoclast precursors into mature osteoclasts, which leads to increased osteoclastic activity that may result in peri-implant bone resorption.23 Furthermore, the additional expression of matrix metalloproteinase (MMP-8) leads to an expanding zone of soft-tissue damage and inflammation that, given time, spreads to the adjacent bone.20 In particular, foreign particles that range from 0.2 µm to 7.2 µm in size have been classified as highly pro-inflammatory.24-26
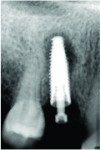
When the exposure of implant threads to the oral environment results in subsequent bacterial colonialization, it is often described as "the beginning of a bad ending" as peri-implant disease accelerates. This leads to crestal bone loss and radiographic translucencies. Carbonaceous contaminants in the apical region of implants may well lead to bone resorption that appears in radiographs like a periapical lesion (Figure 12). An additional potential threat to healing following implant placement is posed by thin-layered residues of highly aggressive cell-toxic cleaning agents such as dodecylbenzene sulfonic acid or quaternary ammonium compounds (ie, biocides) on the surfaces of some brand-new implants (Figure 9). Even in low concentrations, these chemicals are directly cytotoxic to cells and do not promote implant healing but rather hamper it.
All the implants in the CleanImplant Foundation's quality assessment studies that were found to have significant impurities were cleared by the US Food and Drug Administration or Health Canada's HPFB or carried the European Commission's CE mark. Ultimately, contaminated implants, even in sterile packaging, can be harmful to patients and lead to implant failure stemming from inflammatory reactions to the impurities.
Discussion
The immunological response to contaminants on dental implants is patient dependent. Some patients will exhibit minimal or no reaction whereas others will exhibit extreme reactions. Nonetheless, the increasing identification of peri-implant diseases due to improved clinical understanding demonstrates that contaminants may be resulting in immunological reactions in many patients.
Contaminants on the surface of an implant constitute a contaminated implant. This is not a difficult problem to solve. Implant manufacturers have the means to prevent this. It can be done, and there is no excuse for it not being done. They owe this to clinicians, to patients, and to themselves. To protect the health of our patients, the science behind the materials that we use warrants unprecedented and unimpeachable quality standards. As a health science profession, dentistry must foster trust within the circle of caregiving-that is the ultimate reward of service.
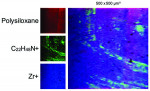
In the CleanImplant Foundation's studies, particulate and thin film contaminants on implant surfaces are identified using a unique combination of analytic techniques. The precise location of impurities is detected using SEM with additional elemental analyses by energy-dispersive X-ray spectroscopy (EDS) performed in a particle-free clean room. Then, the chemical composition of these contaminants is identified by subsequent time-of-flight secondary ion mass spectrometry (ToF-SIMS). Both analytical tasks are performed in accredited testing laboratories according to DIN EN ISO/IEC 17025:2018, which ensures that all analyses are precise and unbiased.
When an implant is not immediately loaded, crestal bone loss following placement can be related to contaminants at the implant's shoulder leading to a localized immunological reaction and progressing to bone loss. Apical bone loss around an implant is not a usual clinical presentation after placement. Prior to prosthetic loading, if apical bone loss is observed in healed sites demonstrating osseointegration, the inflammatory process may be the result of previous endodontic infection at the site. However, it may also be the result of surface contamination that occurred during manufacturing and/or subsequent sterile packaging. As the use of dental implants continues to increase globally, patients receiving these devices need to be carefully monitored throughout the lifetimes of their restorations. Early identification of peri-implant mucositis and intervention is key to preserving the bone surrounding implants, preventing progression to peri-implantitis, and improving long-term clinical results.
Implant surface quality, and more specifically, the implant's cleanliness, may play a major and still underrated role in peri-implant disease. Whether implants are titanium or ceramic, their surfaces must be free of foreign particles after they are removed from the sterile packaging. Such particles are ultra-small and not visible to the naked eye or with loupes or operatory microscopes. In most cases of peri-implantitis or implant failure, clinicians assume that the problem lies with the patient. However, the results of quality assessments of sterile-packaged implants as cited earlier suggest that the implants themselves should also be considered as possible triggers for inflammatory reactions and potential causes of peri-implantitis following insertion. Clinicians should be assured that the manufacturing and packing of their chosen dental implant systems results in implants that are pristine, uncontaminated, and safe to use on patients. In the absence of such assurances from manufacturers, a thorough SEM analysis of implants taken from various batches from clinicians' inventories can provide sufficient documented proof of a system's cleanliness.27-29 As Norton expressed, "All implants are not the same, and the consequences of ‘look-alike' implants relying on the documentation of others may be far-reaching."30 We get what we pay for, and companies that offer discount implants may be cutting corners in the manufacturing and packaging process to improve profits.
Conclusion
Despite best manufacturing and packaging practices, contaminants and pollutants have been found on the surfaces of sterile-packaged implants, and the presence of such contaminants and pollutants at the bone-implant interface can initiate an inflammatory response with consequential bone resorption. There is a pressing need for the industry to recognize the inherent value of screening dental implant devices for these toxic compounds to obviate the bio-interface reactions that they can cause during the early phase of osseointegration.
For the past 8 years, the CleanImplant Foundation has worked with a growing group of industry partners to ensure particle-free implant production. It has introduced a quality seal for tested, verified clean implants, the foundation's "Trusted Quality Mark," which is awarded by its scientific advisory board through a peer-reviewed process that involves a rigorous, independent analysis of five randomly selected samples from the implant system every 2 years.
Disclosure
Scott D. Ganz, DMD, is a member of the CleanImplant Foundation's scientific advisory board, Dirk U. Duddeck, DDS, is the founder and managing director of the CleanImplant Foundation, and Kenneth S. Serota, DDS, MMSc, is the North American representative of the CleanImplant Foundation. Gregori M. Kurtzman, DDS, had no disclosures to report.
About the Authors
Scott D. Ganz, DMD
Adjunct Assistant Professor
Department of Restorative Dentistry
Rutgers University
School of Dental Medicine
Newark, New Jersey
Co-Director
Advanced Implant Education
Private Practice
Fort Lee, New Jersey
Dirk U. Duddeck, DDS
Guest Researcher
Charité University Medicine
Berlin, Germany
Managing Director and Head of Research
CleanImplant Foundation
Berlin, Germany
Gregori M. Kurtzman, DDS
Master
Academy of General Dentistry
Diplomate
International Congress of Oral
Implantologists
Private Practice
Silver Spring, Maryland
Kenneth S. Serota,
DDS, MMSc
North American Representative
CleanImplant Foundation
References
1. Astolfi V, Ríos-Carrasco B, Gil-Mur FJ, et al. Incidence of peri-implantitis and relationship with different conditions: a retrospective study. Int J Environ Res Public Health. 2022;19(7):4147.
2. Heitz-Mayfield L. Peri-implant mucositis and peri-implantitis: key features and differences. Br Dent J. 2024;236:791-794.
3. Scarano A, Khater AGA, Gehrke SA, et al. Current status of peri-implant diseases: a clinical review for evidence-based decision making. J Funct Biomater. 2023;14(4):210.
4. Lo Bianco L, Montevecchi M, Ostanello M, Checchi V. Recognition and treatment of peri-implant mucositis: do we have the right perception? A structured review. Dent Med Probl.2021;58(4):545-554.
5. Greenstein G, Eskow R. High prevalence rates of peri-implant mucositis and peri-implantitis post dental implantations dictate need for continuous peri-implant maintenance. Compend Contin Educ Dent.2022;43(4):206-213.
6. Hu C, Lang NP, Ong MM, et al. Influence of periodontal maintenance and periodontitis susceptibility on implant success: a 5-year retrospective cohort on moderately rough surfaced implants. Clin Oral Implants Res. 2020;31(8):727-736.
7. Lee CT, Huang YW, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent. 2017;62:1-12.
8. Astolfi V, Rios-Carrasco B, Gil-Mur FJ, et al. Incidence of peri-implantitis and relationship with different conditions: a retrospective study. Int J Environ Res Public Health. 2022;19(7):4147.
9. Diaz P, Gonzalo E, Villagra LJG, et al. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health. 2022;22(1):449.
10. Schwarz F, Derks J, Monje A, Wang HL. Peri-implantitis. J Periodontol. 2018;89(Suppl 1):S267-S290.
11. Froum S, Kurtzman GM. Top 5 anatomical differences between dental implants and teeth that influence treatment outcomes. Perio Implant Advisory website. https://www.
perioimplantadvisory.com/clinical-tips/article/16412223/top-5-anatomical-differences-between-dental-implants-and-teeth-that-influence-treatment-outcomes. Published November 9, 2021.
12. Dhir S, Mahesh L, Kurtzman GM, Vandana KL. Peri-implant and periodontal tissues: a review of differences and similarities. Compend Contin Educ Dent.2013;34(7):e69-e75.
13. Amerio E, Blasi G, Valles C, et al. Impact of smoking on peri-implant bleeding on probing. Clin Implant Dent Relat Res. 2022;24(2):151-165.
14. Costa FO, Lages EJP, Cortelli SC, et al. Association between cumulative smoking exposure, span since smoking cessation, and peri-implantitis: a cross-sectional study. Clin Oral Investig. 2022;26(7):4835-4846.
15. Renvert S, Polyzois I. Risk indicators for peri-implant mucositis: a systematic literature review. J Clin Periodontol. 2015;42(Suppl 16):S172-S186.
16. Lee DW. Periodontitis and dental implant loss. Evid Based Dent.2014;15(2):59-60.
17. Zahra B, Nicholas B, Geoffrey R, et al. Dental implant failure rates in patients with self-reported allergy to penicillin. Clin Implant Dent Relat Res. 2022;24(3):301-306.
18. Ganz SD, Duddeck DU, Kurtzman GM. Peri-implantitis and the effect of the implant surface at placement. Compend Contin Educ Dent. 2023;44(1):52-55.
19. Duddeck DU, Albrektsson T, Wennerberg A, et al. On the cleanliness of different oral implant systems: a pilot study. J Clin Med.2019;8(9):1280.
20. Marahleh A, Kitaura H, Ohori F, et al. TNF-α directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front Immunol. 2019;10:2925.
21. Duddeck DU, Albrektsson T, Wennerberg A, et al. Quality assessment of five randomly chosen ceramic oral implant systems: cleanliness, surface topography, and clinical documentation. Int J Oral Maxillofac Implants.2021;36(5):863-874.
22. Nicolas-Silvente AI, Velasco-Ortega E, Ortiz-Garcia I, et al. Influence of the titanium implant surface treatment on the surface roughness and chemical composition. Materials (Basel). 2020;13(2):314.
23. Mouhyi J, Dohan Ehrenfest DM, Albrektsson T. The peri-implantitis: implant surfaces, microstructure, and physicochemical aspects. Clin Implant Dent Relat Res. 2012;14(2):170-183.
24. Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis.2009;67(2):182-188.
25. Matthews JB, Besong AA, Green TR, et al. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000;52(2):296-307.
26. Rader CP, Sterner T, Jakob F, et al. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J Arthroplasty. 1999;14(7):840-848.
27. Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67(2):182-8.
28. Shanbhag AS, Bailey HO, Hwang DS, et al. Quantitative analysis of ultrahigh molecular weight polyethylene (UHMWPE) wear debris associated with total knee replacements. J Biomed Mater Res. 2000;53(1):100-110.
29. Duddeck D, Albrektsson T, Wennerberg A, et al. CleanImplant Trusted Quality Mark 2017 -2018: Process Description Quality Mark Criteria. CleanImplant Foundation website. https://www.cleanimplant.com/Awards/implants/index.php/. Published September 19, 2017. Accessed August 9, 2024.
30. Norton MR. Will any dental implant do? Br Dent J.2020;228(4):243-244.