Systemic and Local Antibiotics and Host Modulation in Periodontal Therapy: Where are we Now? Part I
Francis G. Serio, DMD, MS, MBA, and Cheryl L. Serio, DDS, FAGD
Nonsurgical therapy is the cornerstone of periodontal care. Typically, this mode of treatment includes a complete periodontal examination, formulation of diagnoses, development of a comprehensive treatment plan, oral hygiene instruction and execution, removal of supragingival and subgingival plaque and calculus, the correction of local plaque-retentive factors, and maintenance care. Advanced nonsurgical therapy may be instituted when a patient has had an inadequate response to mechanical nonsurgical therapy and perhaps surgical care as well. Advanced nonsurgical therapy includes the use of systemic and local antibiotics and the recently developed host modulation modalities. This article will examine the biological and clinical aspects of systemic antibiotic therapy. Part 2 will discuss the use of local antibiotic and chemotherapeutic delivery and alteration of the host response to the challenge of periodontal pathogens.
THE ETIOLOGY OF THE PERIODONTAL DISEASES
It is now well-established that bacterial plaque is associated with the initiation and progression of the inflammatory periodontal diseases. While much work has been done to fully understand the composition, organization, and influence of bacterial colonies on the health of the periodontal tissues,1,2 some basic questions remain.3
Because the etiology of the periodontal diseases is quite complex, Henle-Koch’s postulates have not been satisfied. These postulates were developed for monoinfections and it is clear that the cause and progression of periodontal disease is multifactorial in nature. Socransky and colleagues4,5 have suggested an alternative hypothesis to identify periodontal pathogens:
- The major portion of the target bacteria should be associated with periodontitis;
- Elimination of target bacteria will result in the cessation of disease progression;
- Host response against the target bacteria should be elucidated;
- If possible, animal models should be used to determine pathogenicity;
- Possible unique mechanisms of pathogenicity should be indicated.
Although there is much information on the bacteria associated with periodontal disease, the specific bacteria that cause disease initiation and progression have yet to be identified. The transition from gingivitis to periodontitis is also poorly understood. Recently, it has been suggested that spirochetes play a much more important role than previously thought. Viruses, particularly the herpes virus, have been implicated in the appearance of periodontal infections.3 Other microorganisms may contribute to this transition as well.6
The progressive growth and change of plaque on root surfaces was demonstrated by electron microscopy by Listgarten.7,8 He described patterns of bacterial colonization based on microscopic appearance. More recently, bacterial colonization has been defined in general and specific terms. “Healthy” plaque has been characterized as being predominantly gram-positive, nonmotile, with facultative aerobic capabilities. Pathogenic bacteria have been generally categorized as gram-negative, more motile, with strict anaerobic respiration.
The colonization of bacteria on tooth surfaces and in subgingival areas is now known as a biofilm.5,9 By definition, a biofilm is defined as a structured community of bacterial cells enclosed in a self-produced polymeric matrix.10,11 The understanding of the organization and function of biofilm is critical to understanding the efficacy of systemic antibiotic therapy. Biofilm is highly organized, providing a protective barrier for the species deep in the bacterial mat to multiply rapidly and survive. Many of the species in the biofilm interact synergistically to provide mutual support. The polymeric glycocalyx provides protection from the effects of systemic antibiotics in the tissues. In addition, the structure of the biofilm contains many channels for nutrient and waste transport from one area to another. In a clean area, supragingival biofilm formation commences, matures, and contributes to the formation of the subgingival biofilm.12 Once the subgingival biofilm has been established, it may exist independent of the presence or absence of supragingival plaque.
Subgingival plaque, which is associated with destructive periodontal inflammation, may exist as unattached plaque, attached plaque, or it may be associated with the epithelium. Biofilms generally form on fixed surfaces such as the root of a tooth. Unattached plaque may be found deep within a periodontal pocket adjacent to the pocket epithelium. It is within the unattached plaque that the main periodontopathogens that have been termed the “red complex”—Porphyromonas gingivalis, Tannerella forsythensis (formerly Bacteroides forsythus), and Treponema denticola—may be found.2,13 Actinobacillus actinomycetemcomitans, the predominant organism associated with aggressive periodontitis (formerly called juvenile periodontitis), may also be found in the unattached plaque and invades the gingival soft tissue all the way to bone. Figure 1 summarizes the current understanding of the pathogenicity of dental plaque.5
ANTIBIOTICS USEFUL IN PERIODONTAL THERAPY
The use of systemic antibiotics to control periodontal disease has attracted great interest during the past several years. Several antibiotics are currently suggested for use (Table 1),14 including amoxicillin with or without clavulanic acid (Augmentin®, GlaxoSmithKline, Research Triangle Park, NC), metronidazole, azithromycin, ciprofloxacin, clindamycin, tetracycline, doxycycline, and subantimicrobial dose doxycycline.
BACTERIAL TESTING
There are three approaches to the selection of systemic antibiotics for the treatment of periodontal infections.15 Culture and antibiotic sensitivity testing has been held as the gold standard for microbial identification. The major drawback to culturing is the necessity of having live bacteria to culture. It is imperative that subgingival samples be minimally exposed to air and placed in a lab-supplied reducing media immediately. Samples must be shipped to the lab by overnight delivery to maximize the viability of the bacteria. One lab has advised its customers that it will not accept specimens shipped on a Thursday or Friday (Oral Microbiology Testing Service, Temple University, written communication, June 2005). It is important to remember that the lab report identifies only those bacteria that were able to be grown in culture, not necessarily all of the species existing in the sample.
A second approach is to use DNA probe analysis. The major advantage to this technique is that bacteria need not be viable. Reporting is limited to the species of bacteria for which the lab has DNA probes, albeit the usual periodontal pathogens are tested. The most commonly tested pathogens are A actinomycetemcomitans, Campylobacter rectus, Eikenella corrodens, Fusobacterium nucleatum, Peptostreptococcus micros, P gingivalis, Prevotella intermedia, T forsythensis, and T denticola. One laboratory asserts that antibiotic sensitivity is not necessary because most pathogens are susceptible to the same constellation of antibiotics.16 Table 2 lists laboratories that conduct culture and sensitivity testing and DNA probe analysis.
The third approach is to prescribe the antibiotics empirically, based on clinical experience and/or the patient’s medical history and sensitivity to the desired antibiotics. The rationale supporting this approach is similar to that above, that most pathogens are susceptible to the same antibiotics. Identification of specific bacteria is reserved for those situations where there is no or minimal clinical improvement after a course of systemic antibiotics or to ensure the elimination of the target bacteria.
CLINICAL APPLICATIONS
There is general agreement that systemic antibiotics are not indicated to treat the vast majority of cases of chronic periodontitis. Most cases respond quite well to conventional nonsurgical and surgical therapy. One systematic review suggests that systemic antibiotics may be useful in treating everyday chronic periodontitis, although the variability in the reviewed studies made definitive conclusions difficult.17
When, then, are systemic antibiotics indicated? The most clear-cut case is the patient with aggressive periodontitis. As mentioned above, A actinomycetemcomitans cannot be eradicated by mechanical therapy alone. In this instance, amoxicillin in combination with metronidazole is an effective approach.18 Doxycycline has also been used effectively againstthis pathogen.
In cases of chronic periodontitis, antibiotics are most often used when the patient has not responded to conventional therapy, the so-called refractory case, or in instances of recurrent disease. Even after thorough mechanical root instrumentation and expert plaque control, the patient still exhibits signs of inflammation and perhaps progressive bone and attachment loss. Although there is wide variationin antibiotic protocols, one successful approach is to use Augmentin in combination with metronidazole. Some researchers maintain that the clavulanic acid is not necessary, but there is more than a passing chance that there is significant presence of B-lactamase in the biofilm. The specific recommendation is the responsibility of the individual practitioner.19-23
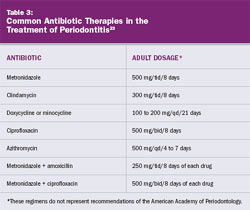
In some instances, the patient may have severe inflammation, erythema, and edema in greater amounts than would be expected based on the physical presence of etiologic factors. Based on clinical experience, the combination of systemic antibiotics and aggressive and thorough scaling and root planing often exhibit dramatic results. This approach is helpful when treating patients who must travel great distances for care and may not be easily accessible for frequent follow-up appointments. Table 3 lists common antibiotic therapies in the treatment of periodontitis.
SEQUENCE OF THERAPY
The proper therapeutic sequence is dictated by the nature of biofilms. As discussed, intact biofilms may form a protective barrier for organisms deep in the community. It is imperative that the biofilm be thoroughly disrupted at the initiation of systemic antibiotic therapy. If it is not possible to complete a full-mouth debridement in one visit, multiple visits within a few days will allow the antibiotics to have maximum exposure to a greatly reduced biofilm. This increased efficacy will result in optimal healing in the shortest possible time.
CONCLUSION
Systemic antibiotics are a useful and effective adjunct to conventional periodontal therapy. Although their use is limited in chronic periodontitis, systemic antibiotics are used routinely in cases of aggressive periodontitis due to the penetration of A actinomycetemcomitans into the gingival tissues. Clinicians should be mindful of the characteristics of biofilms to have realistic expectations of theperformance of antibiotics in the absence of biofilm disruption.
References
1. Haffajee AD, Socransky SS. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78-111.
2. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135-187.
3. Slots J. Herpesviruses in periodontal infections. Periodontol 2000. 2005;38:33-62.
4. Socransky SS. Microbiology of periodontal disease—present status and future considerations. J Periodontol. 1977;48(9): 497-504.
5. Nishihara T, Koseki T. Microbial etiology of periodontitis. Periodontol 2000. 2004;36:14-26.
6. Ellen RP, Galimanas VB. Spirochetes at the forefront of periodontal infections. Periodontol 2000. 2005;38:13-32.
7. Listgarten MA. The structure of dental plaque. Periodontol 2000. 1994;5:52-65.
8. Listgarten MA. Structure of surface coatings of teeth. A review. J Periodontol. 1976;47(3):139-147.
9. Socransky SS, Haffajee AD. Dental biofilms: difficult therapeutic targets. Periodontol 2000. 2002;28:12-55.
10. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause ofpersistent infections. Science. 1999;284(5418):1318-1322.
11. Costerton JW, Lewandowski Z, Caldwell DE, et al. Microbial biofilms. Annu Rev Microbiol. 1995;49:711-745.
12. Marsh PD. Dental plaque: biological significance of a biofilm and community life-style. J Clin Periodontol. 2005;32(suppl 6):7-15.
13. Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the ‘red complex’, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72-122.
14. Wynn RL, Meiller TF, Crossley HL. Drug Information Handbook for Dentistry. 11th ed. Hudson, OH: Lexi-Comp; 2006.
15. van Winkelhoff AJ, Winkel EG. Microbial diagnostics in periodontics: biological significance and clinical validity. Periodontol 2000. 2005;39: 40-52.
16. Microdentex. DMDx Testing Kit Susceptibility Charts. Available at: www.microdentex.com/pro_dmdx_susceptibilitycharts.asp. Accessed January 3, 2006.
17. Herrera D, Sanz M, Jepsen SJ, et al. A systematic review on the effects of systemic antimicrobials as an adjunct to scaling and root planing in periodontal patients. J Clin Periodontol. 2002;29(suppl 3):136-159.
18. Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonasgingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999;20: 82-121.
19. Slots J. Selection of antimicrobial agents in periodontal therapy. J Periodont Res. 2002;37(5):389-398.
20. Slots J, Ting M. Systemic antibiotics in the treatment of periodontal disease. Periodontol 2000. 2002;28:106-176.
21. Haffajee AD, Socransky SS, Gunsolley JC. Systemic anti-infective periodontal therapy. A systematic review. Ann Periodontol. 2003;8(1):115-181.
22. Walker CB, Karpinia K, Baehni P. Chemotherapeutics: antibiotics and other antimicrobials. Periodontol 2000. 2004;36:146-165.
23. Slots J. Research, Science and Therapy Committee. Systemic antibiotics in periodontics. American Academy of Periodontology Position Paper. J Periodontol. 2004;75(11):1553-1565.
 Figure 1 Subgingival plaque as a pathogenic biofilm (figure reprinted with permission from Periodontal 20005).  |
  |
|
| About the Authors | ||
 Francis G. Serio, DMD, MS, MBA Francis G. Serio, DMD, MS, MBAProfessor and Chairman Department of Periodontics and Preventive Sciences University of Mississippi School of Dentistry Jackson, Mississippi |
||
 Cheryl L. Serio, DDS, FAGD Cheryl L. Serio, DDS, FAGDAssistant Professor Department of Care Planning and Restorative Sciences University of Mississippi School of Dentistry Jackson, Mississippi |
||





