Abstract:
OBJECTIVE: The objective of this study was to evaluate the effect of a dental water jet on plaque biofilm removal using scanning electron microscopy (SEM).
METHODOLOGY: Eight teeth with advanced aggressive periodontal disease were extracted. Ten thin slices were cut from four teeth. Two slices were used as the control. Eight were inoculated with saliva and incubated for 4 days. Four slices were treated using a standard jet tip, and four slices were treated using an orthodontic jet tip. The remaining four teeth were treated with the orthodontic jet tip but were not inoculated with saliva to grow new plaque biofilm. All experimental teeth were treated using a dental water jet for 3 seconds on medium pressure.
RESULTS: The standard jet tip removed 99.99% of the salivary (ex vivo) biofilm, and the orthodontic jet tip removed 99.84% of the salivary biofilm. Observation of the remaining four teeth by the naked eye indicated that the orthodontic jet tip removed significant amounts of calcified (in vivo) plaque biofilm. This was confirmed by SEM evaluations.
CONCLUSION: The Waterpik® dental water jet (Water Pik, Inc, Fort Collins, CO) can remove both ex vivo and in vivo plaque biofilm significantly.
Scientific technology has expanded the profession’s understanding of dental plaque. Treatment and prevention are now focused on dental plaque as a biofilm. Biofilms are three-dimensional arrangements of bacteria that are loosely or more firmly adherent to teeth and tissue. Biofilms consist of microcolonies of bacteria embedded in slimy matrices. Biofilms are self-sufficient, dynamic communities that can survive in hostile environments. The regular removal of dental plaque biofilm, which contain the bacteria responsible for caries formation and for the etiology of gingivitis and periodontitis, is the well-accepted sine qua non of dental health.
In other ecosystems in which biofilms harbor bacteria that attack surfaces, such as steel,1 two basic strategies of biofilm control have emerged.2 The first is predicated on the use of chemicals to kill the bacteria in the biofilm to induce the natural sloughing of dead biofilm, thus cleaning the surface and preventing corrosion.3 The second is to remove the matrix-enclosed bacterial microcolonies from the surface by the use of shear forces that overcome the tensile strength of the matrix material without damaging the integrity of the material’s surface. The chemical approach suffers from the limitation that the most effective antimicrobial agents do not penetrate the biofilm, so it is very difficult to deliver enough of the agent to clean the surface, and the biofilm can return to its original state easily. The physical removal of biofilm from surfaces cleans the surfaces very effectively,4 and removes the insidious bacteria from the system completely. Shear forces are widely used to clean oil and water pipelines, and the same is true for dental biofilms. Mechanical removal is the most effective method to control the growth of biofilm. Biofilms are accessible to a dental professional and can be effectively removed by scaling and root planing. It is more difficult for patients to effectively remove or disrupt the biofilm from all surfaces of the tooth on a daily basis.
The dental water jet has been studied extensively for the past 45 years. The research demonstrates that a combination of 1,200 pulsations per minute and pressure settings of 55 psi to 90 psi are safe and can significantly reduce bleeding and gingivitis in a variety of cohorts. Clinical studies of inflammation have shown statistically significant repeatable improvement with the use of the water jet,5-19 but erythrosine-based plaque indices have yielded equivocal data. Some studies have shown a reduction in the plaque index with the use of the water jet compared with a control,7,8,12,18,19 while other studies have shown no significant differences.5,14-17 The impact of a dental water jet on the quality and quantity of supragingival plaque biofilm remains essentially unknown. A few studies have examined the supra- and subgingival biofilm microscopically.
Brady and colleagues20 examined the impact of a dental water jet on the ultrastructure of supragingival dental biofilm on rhesus monkeys with an electron microscope. Experimental sites were treated with a pulsating water jet at a pressure setting of 70 psi. Posttreatment biofilm samples showed either removal of biofilm or irreversible damage to the bacteria in the biofilm matrix compared with untreated sites.20 Cobb et al21 found similar results in human patients. Periodontally involved teeth were treated with water irrigation at a pressure of 60 psi and then extracted with the epithelial lining intact. The treated sites showed few cocci and short rods randomly dispersed and associated with a light fibrin-like matrix. In contrast, the untreated controls exhibited thick mattes of organisms (short rods, long fusiforms, and chains of cocci), including spirochetes.21 Other studies have evaluated the reduction of specific subgingival organisms and have shown a significant reduction in Prevotella intermedia,5 Bacteroides species,13 and spirochetes22 in 4-mm to 6-mm pockets.
This study evaluated the hydraulic forces (shear forces) produced by a pulsating dental water jet (Water Pik, Inc, Fort Collins, CO) on ex vivo and in vivo biofilm using scanning electron microscopy (SEM).
Methods and Materials
Eight teeth were extracted from a patient with advanced aggressive periodontitis. Institutional Review Board approval was obtained (proposal No. IR00000792), as well as informed consent from the patient. The teeth were fixed in Karnovsky’s solution23 for 48 hrs at 4°C and washed twice in phosphate-buffered saline. Ten thin slices comprising the regions spanning above and below the cementoenamel junction were cut from four of the extracted teeth and sterilized by autoclaving. The cut slices were placed in two 6-well plates and filled with 6 mL of Todd-Hewitt media. Saliva was taken from a volunteer and incubated in Todd-Hewitt media for 24 hrs at 37°C. The two 6-well plates containing the tooth slices were inoculated with the precultured salivary biofilm (ex vivo) and incubated for 4 days at 37°C with daily media change. Eight of the tooth slices were mounted individually on a clamp. The dental water jet was used in accordance with the manufacturer’s instructions for the standard jet and the orthodontic jet tip. The unit was set on a medium-pressure setting of 6 ( ˜70 psi). Each sample was treated for 3 seconds and timed using a digital metronome (Metrina Multi 353, Zen-On Music, Co, Ltd, Tokyo, Japan) set to 120 (two beats per second). Four tooth slices were treated with the standard jet tip (Figure 1A), and four tooth slices were treated with the orthodontic jet tip (Figure 1B). Two tooth slices with ex vivo-grown salivary biofilm served as controls. The 10 treated and untreated slices with ex vivo salivary biofilms were examined by SEM. The four remaining extracted teeth were treated with the orthodontic jet tip to evaluate the effect on in vivo calcified biofilm. No additional salivary biofilm was grown on these teeth as described previously. The four samples with in vivo-calcified biofilm were evaluated with the naked eye and SEM.
Scanning Electron Microscopy
The treated and untreated tooth slices were dehydrated in graded ethanol, critical point-dried with carbon dioxide, and mounted on a stub. The samples were sputter-coated with 25 nm platinum and examined with a scanning electron microscope with 5 KeV in the secondary electron mode (XL 30 S, FEG, Philips/FEI Co, Hillsboro, OR).
Images of the control and samples were taken in the SEM from representative areas of treated and untreated regions of the tooth slices, and total bacteria numbers were counted on standard areas of 10 µm x 10 µm. The mean was determined, and the results were extrapolated on a standard area of 1 cm2. The extrapolated area was then multiplied with the number of bacterial layers of the biofilm. The total bacterial load was calculated. Because of the simplistic assumptions (exact determination of the tooth surface, number of biofilm layers, and even distribution), this calculation can be regarded only as a semi-quantitative approximation of the number of bacteria in the biofilm.24
Results
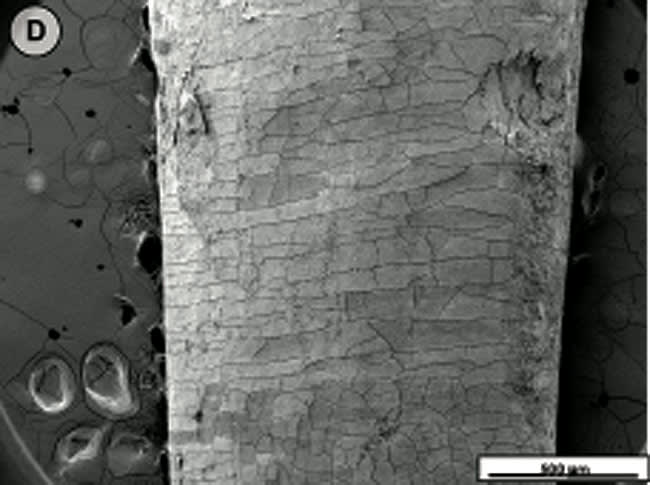
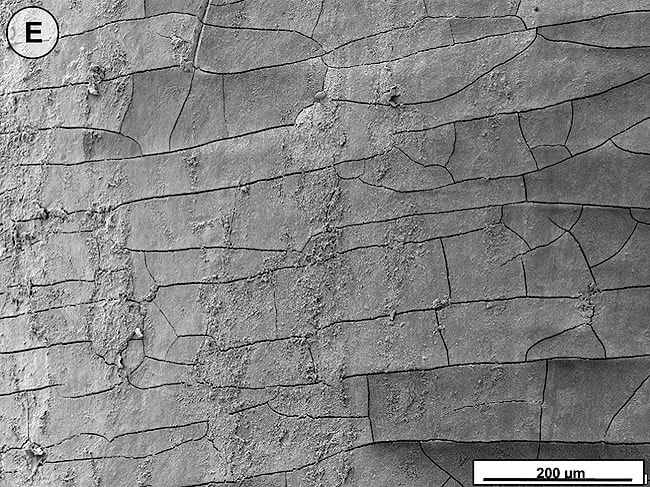
When the tooth slices with the ex vivo-grown salivary biofilm were examined under the scanning electron microscope, they were colonized by luxuriant biofilm covering the entire surface (Figure 2A, Figure 2B, Figure 2C). The biofilms appeared to be several micrometers thick. The predominant morphotypes in the biofilms were fusiform bacteria and cocci. Several regions showed co-aggregation between the two morphotypes, which is a phenomenon of mutual dependence for nutrition and growth. The salivary-derived biofilm showed characteristics typical of a naturally occurring in vivo biofilm in the mouth. The standard jet tip treatment for 3 seconds on the tooth slices with ex vivo-grown biofilm showed extensive areas of biofilm removal in comparison with the untreated control slices (Figure 2D, Figure 2E, Figure 2F). The standard jet removed 99.99% of the salivary biofilms. The orthodontic tip treatment for 3 seconds on the tooth slices appeared to clear very extensive areas of ex vivo-grown salivary biofilm (Figure 3A andFigure 3B). Biofilm removal was observed both at the crown surface and below the cementoenamel junction. The percentage of biofilm removed by the orthodontic tip was 99.84%. Observation with the naked eye indicated that treatment of in vivo biofilm with the orthodontic tip removed significant amounts of this calcified biofilm. This was evident in SEMs, which showed the presence of clearance marks (Figure 3C) caused by the bristles associated with this tip.
Discussion
A high level of confidence can be placed in the direct demonstration of the removal of biofilm by microscopic methods,25 in contrast with other studies that have used scraping for recovery and plating techniques for the enumeration of sessile bacteria.26 This confidence can be assured because of a recent demonstration27 that bacterial cells in biofilm grow poorly, if at all, when they are placed on the surfaces of agar plates, so that the enumeration of biofilm bacteria by scraping and plating is not valid. This study approached the real situation in the oral environment, in that the removal of biofilm from well-defined regions of the surfaces of extracted teeth was compared with untreated regions of the same tooth and untreated controls. The teeth used in this study were extracted from a patient with severe periodontitis, so that supragingival and subgingival biofilm was available for evaluation and was the ideal surface for growing ex vivo salivary biofilm. The data presented here demonstrate that a 3-second exposure to hydraulic forces produced by a pulsating water stream from a dental water jet with 1,200 pulsations per minute exerting shear force ( ˜70 psi) removed biofilm from the tooth surface both above and below the cementoenamel junction with 99.99% and 99.84% efficiency.
Comparing dental biofilm against the whole spectrum of biofilm studied by biofilm engineers, dental biofilm’s susceptibility to removal by shear forces fits into a logical pattern. Microbial biofilms have been shown to vary the cross-linking of the component polymers of their matrices to develop a tensile strength appropriate for their retention on surfaces in the ecosystem in which they operate. Various degrees of mineralization of biofilms make them much more resistant to removal by shear forces. In the oral ecosystem, mineralization takes the form of calcification, and the deeper layers of the biofilm used in this study were, in fact, calcified to the extent that they had tensile strengths approaching that of the enamel of the tooth. For this reason, the authors distinguished between the removal of less calcified ex vivo salivary biofilm and the removal of calcified biofilm that had formed over a long period on the patient’s teeth in vivo.
Recent published clinical studies measuring the use of water with either the orthodontic tip or standard jet tip on biofilm removal have used traditional plaque biofilm indices. A randomized clinical study comparing a dental water jet with the orthodontic tip plus manual toothbrushing with manual toothbrushing and flossing or manual toothbrushing alone showed a significantly greater reduction in biofilm for the dental water jet group compared with flossing (3.76 times) or manual toothbrushing (5.83 times) in adolescent patients with fixed orthodontic appliances.18 A dental water jet paired with either manual or sonic toothbrushing showed a greater reduction in biofilm removal compared with manual toothbrushing and flossing.6 The differences were significant for sonic toothbrushing and dental water jet use compared with manual tooth- brushing and flossing. A 2-week study demonstrated a significantly greater reduction in biofilm with the standard jet tip use compared with routine oral hygiene practices.8
This microscopic study adds to the existing data and provides an explanation for the consistent reduction in inflammation from using a dental water jet. Along with biofilm removal, other studies have shown reductions in the subgingival microflora,21,22 changes in the cells resulting in decreased viability and cell death,20,21 and a reduction in the serum and gingival crevicular fluid measures of pro-inflammatory mediators.7,8
Conclusion
This study demonstrated microscopically that the hydraulic forces produced by a dental water jet with 1,200 pulsations per minute on medium pressure (˜70 psi) (Water Pik, Inc) can significantly remove biofilm from tooth surfaces above and below the cementoenamel junction in vitro. A standard jet tip can remove 99.99% of ex vivo-grown biofilm with 3 seconds of use. An orthodontic tip can remove 99.84% of ex vivo-grown biofilm with 3 seconds of use. And, an orthodontic tip can remove in vivo-grown biofilm significantly with 3 seconds of use, as observed by the naked eye and SEM.
References
1. McCoy WF, Bryers JD, Robbins J, et al. Observations of fouling biofilm formation. Can J Microbiol. 1981;27(9):910-917.
2. Lee W, Lewandowsky Z, Nielsen PH, et al. Role of sulphate-reducing bacteria in corrosion of mild steel: a review. Biofouling. 1995:8:165-94.
3. Colturi TF, Kozelski KJ. Corrosion and biofouling control in a cooling tower. Material Performance. 1984;23(8):43-47.
4. Ridgway HF, Flemming HC. Membrane biofouling. In: Mallevialle J, Odendaal PE, Wiesner MR, eds. Water Treatment Membrane Processes. New York, NY: McGraw-Hill; 1996:6.1-6.62.
5. Chaves ES, Kornman KS, Manwell MA, et al. Mechanism of irrigation effects on gingivitis. J Periodontol. 1994;65(11):1016-1021.
6. Barnes CM, Russell CM, Reinhardt RA, et al. Comparison of irrigation to floss as an adjunct to tooth brushing: effect on bleeding, gingivitis, and supragingival plaque. J Clin Dent. 2005; 16(3):71-77.
7. Al-Mubarak S, Ciancio S, Aljada A, et al.. Comparative evaluation of adjunctive oral irrigation in diabetics. J Clin Periodontol. 2002;29(4):295-300.
8. Cutler CW, Stanford TW, Abraham C, et al. Clinical benefits of oral irrigation for periodontitis are related to reduction of pro-inflammatory cytokine levels and plaque. J Clin Periodontol. 2000;27(2):134-143.
9. Newman MG, Cattabriga M, Etienne D, et al. Effectiveness of adjunctive irrigation in early periodontitis: multi-center evaluation. J Periodontol. 1994;65(3):224-229.
10. Flemmig TF, Newman MG, Doherty FM, et al. Supragingival irrigation with 0.06% chlorhexidine in naturally occurring gingivitis. I. 6 month clinical observations. J Periodontol. 1990; 61(2):112-117.
11. Flemmig TF, Epp B, Funkenhauser Z, et al. Adjunctive supragingival irrigation with acetylsalicylic acid in periodontal supportive therapy. J Clin Periodontol. 1995;22(6):427-433.
12. Felo A, Shibly O, Ciancio SG, et al. Effects of subgingival chlorhexidine irrigation on peri-implant maintenance. Am J Dent. 1997;10(2):107-110.
13. Jolkovsky DL, Waki MY, Newman MG, et al. Clinical and microbiological effects of subgingival and gingival marginal irrigation with chlorhexidine gluconate. J Periodontol. 1990; 61(11):663-669.
14. Brownstein CN, Briggs SD, Schweitzer KL, et al. Irrigation with chlorhexidine to resolve naturally occurring gingivitis. a methodologic study. J Clin Periodontol. 1990;17(8):588-593.
15. Ciancio SG, Mather ML, Zambon JJ, et al. Effect of chemotherapeutic agent delivered by an oral irrigation device on plaque, gingivitis, and subgingival microflora. J Periodontol. 1989;60(6): 310-315.
16. Walsh TF, Glenwright HD, Hull PS. Clinical effects of pulsed oral irrigation with 0.02% chlorhexidine digluconate in patients with adult periodontitis. J Clin Periodontol. 1992;19(4):245-248.
17. Fine JB, Harper DS, Gordon JM, et al. Short-term microbiological and clinical effects of subgingival irrigation with an antimicrobial mouthrinse. J Periodontol. 1994;65(1):30-36.
18. Sharma NC, Lyle DM, Qaqish JG, et al. The effect of a dental water jet with orthodontic tip on plaque and bleeding in adolescent patients with fixed orthodontic appliances. Am J Orthod Dentofacial Orthop. 2008;133(4):565-571.
19. Burch JG, Lanese R, Ngan P. A two-month study of the effects of oral irrigation and automatic toothbrush uses in an adult orthodontic population with fixed appliances. Am J Orthodont Dentofacial Orthop. 1994;106(8):121-126.
20. Brady JM, Gray WA, Bhaskar SN. Electron microscopic study of the effect of water jet lavage devices on dental plaque. J Dent Res. 1973;52(6):1310-1313.
21. Cobb CM, Rodgers RL, Killoy WJ. Ultrastructural examination of human periodontal pockets following the use of an oral irrigation device in vivo. J Periodontol. 1988;59(3):155-163.
22. Drisko CL, White CL, Killoy WJ, et al. Comparison of dark-field microscopy and a flagella stain for monitoring the effect of a Water Pik on bacterial motility. J Periodontol.1987;58(6): 381-386.
23. Karnovsky MJ. A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol. 1965;27:137A.
24. Schaudinn C. Characterization of Subgingival Biofilms in Patients with Aggressive Periodontitis with Transmission Electron Microscopy, Scanning Electron Microscopy and Confocal Laser Scanning Microscopy [thesis]. 2008. Germany: Technical University Berlin.
25. Heersink J, Costerton JW, Stoodley P. Influence of the Sonicare toothbrush on the structure and thickness of laboratory grown Streptococcus mutans biofilms. Am J Dent. 2003:16(2):79-83.
26. Gagnon GA, Slawson RM. An efficient biofilm removal method for bacterial cells exposed to drinking water. J Microb Methods.1999;34(3):203-214.
27. Veeh RH, Shirtliff ME, Petik JR, et al. Detection of Staphylococcus aureus biofilm on tampons and menses components. J Infect Dis. 2003:188(4):519-530.
About the Authors
Amita Gorur, MS
University of Southern California Center for Biofilms
Los Angeles, California
Deborah M. Lyle; RDH, MS
Water Pik, Inc
Fort Collins, Colorado
Christoph Schaudinn, PhD
University of Southern California Center for Biofilms
Los Angeles, California
John W. Costerton, PhD
Founding Director
University of Southern California Center for Biofilms
Los Angeles, California