Improving the Prognosis of Periodontally Involved Teeth at the Time of Extraction of Adjacent Teeth With an Amnion-Chorion Barrier and Bioactive Dentin Graft
Robert A. Horowitz, DDS; and Gregori M. Kurtzman, DDS, MAGD
ABSTRACT
With increased awareness, both in the dental literature and by the general public, of peri-implant disease, a growing trend in dentistry is to save teeth with a "questionable" periodontal prognosis. This prospective study involving such patients was designed to evaluate the effects of combining a bioactive barrier and graft, not on the socket but to augment adjacent periodontal conditions on teeth with severe periodontal bone loss at the time of extraction of an adjacent tooth. Fifteen patients were selected; teeth were extracted, ground, prepared with a pH 11 cleanser, partially demineralized, and made into a graft. This mixture was used to augment socket volume and perform periodontal regenerative surgery. The graft was covered with a bioactive amnion-chorion barrier membrane. Bioactive membranes can stimulate host cells in the surrounding gingival and periosteal tissues to accelerate site closure and healing, simultaneously exerting positive effects on the underlying bone and graft material not observed to the same extent with other membranes. This can improve healing and site regeneration as shown clinically and radiographically in this report. Use of these bioactive barrier membrane and dentin graft materials may have additive effects and provide stimulus for conversion to host bone after site healing. The combination of an amnion-chorion membrane with autologous dentin graft appears to maximize the benefits of the individual materials, improving guided tissue regeneration results and the prognoses of periodontally involved teeth.
Periodontal care is intended to preserve or improve the health of teeth. This becomes challenging when periodontal bone loss leads to the need to extract the affected tooth. If periodontal disease is treated early, therapy is more predictable on the affected and adjacent teeth. Similarly, the literature has shown that peri-implantitis is a higher risk in patients with periodontal disease, which can lead to implant loss.1 The sooner peri-implantitis is identified and treated, the greater the long-term survival of the affected implant(s).2
Collapse of the residual alveolar ridge following tooth extraction without socket grafting is well-documented.3 The degree of site resorption relates to the degree of periodontitis present during the lead-up to the loss of that tooth. Significant bone loss has been reported following extraction when severe periodontitis is present.4 This can be prevented or minimized by performing socket grafting at the time of extraction, thereby maintaining the ridge in all dimensions through the use of various materials.5-7
When being extracted, a non-endodontically treated tooth can be prepared and the dentin used as graft material in the same patient at that surgery. This can increase clinical acceptance and may eliminate potential immunological reactions to packaged graft materials. The debridement of the tooth must be thorough, so as to not include any restorative materials or granulation tissue. While the incidence of immunological reactions with xenograft, allograft, and synthetic materials is low it nonetheless has been reported.8 Dentin graft, derived from the extracted tooth, is autogenous to the patient and not reported to cause immunological reactions, being well accepted by the host during healing and maturation.9 Dentin contains growth factors, including bone morphogenetic protein (BMP).10 These have the potential to augment various stages of the socket and periodontal healing processes. The use of bioactive ground dentin has been validated for socket preservation in different locations in the mouth,11 leading to successful placement and loading of dental implants.12 (The preparation and processing of the tooth and graft material are discussed later in this article.)
Wound healing undergoes three stages.13 The first, lasting just 24 to 72 hours, is critical for the results of the underlying graft material placed. At this stage, the inclusion of platelet-rich fibrin (PRF) aids due to its incorporation of neutrophils and macrophages (M1) in the graft material. This allows host clearing of inflammatory products related to the surgery that are present and could hamper initial healing.14 In the next stage, macrophages switch to M2 phenotype, releasing vascular endothelial growth factor (VEGF) and BMP. Use of an amnion-chorion barrier assists in healing as it stimulates the conversion of M1 macrophages to M2 in the site,15 aiding in bone formation around the graft, whether it is demineralized dentin, allograft, xenograft, or combinations of these materials. In the third phase of healing, site remodeling and its contents occurs.16 Demineralized dentin releases soluble growth factors that counter site resorption during healing, maintaining site volume as it organizes. The host's immune cells sense the autologous partially demineralized dentin, similar to autogenous compact cortical bone, inducing an osteogenic response that results in ankyloses of newly formed bone to dentin surfaces in the graft placed. During remodeling, osteoclasts sense dentin within the graft as bone being replaced by newly formed bone, termed replacement resorption.17 Mineralized dentin autograft mixed with PRF is reported to be effective in preserving post-extraction alveolar ridge dimensions, maintaining the area for future implant placement or as a base for an overlying fixed or removable prosthesis.12 Once placed, dentin graft blended with PRF limits soft-tissue ingrowth into the grafted area during the initial healing period, allowing maintenance of the volume at placement.18
Without significant flap manipulation, surgical grafting of bony defects following extraction of teeth or treatment of periodontal defects can result in incomplete crestal seal of the site. Because this can expose the underlying hard tissue and any graft placed to the oral environment, a barrier membrane is used to protect these areas from exposure. This also prevents soft-tissue downgrowth into the clot or graft before site organization can take place and immature new bone can initially fill the defect. Such membranes are generally inert and not bioactive, unable to attract immune cells capable of modulating the regeneration of an active mucoperiosteum. It is well accepted that periosteum is rich in immune and osteogenic populations that when activated can form lamellar compact bone. Moreover, the periosteum will activate regeneration of periodontal ligament (PDL) at adjacent teeth and underlying alveolar bone.
The bioactive membrane used in the present study was an embryonic decellularized amnion-chorion membrane consisting of a basement membrane matrix that includes collagens, cell attachment proteins, proteoglycans, and growth factors. The embryonic membrane activates regeneration of a mucoperiosteum, facilitating reattachment to neighboring root cementum surfaces allowing regeneration of the PDL and alveolar bone while enhancing stem cell migration.19 Interaction also occurs with autologous dentin particles in any underlying graft material. By assisting in graft maturation, an amnion-chorion membrane prevents connective tissue and epithelial tissue ingrowth into the graft.
Materials and Methods
Fifteen patients were entered in this 18-month prospective study. All patients signed surgical consent forms to participate in the surgical procedure and subsequent evaluations. Teeth were extracted atraumatically using minimally invasive methods. Adjacent teeth that had periodontally poor or worse prognoses were treated with aggressive root planing and ethylenediaminetetraacetic acid (EDTA) or a mild acid solution conditioning. While this process was taking place, the extracted teeth were prepared to become all or part of the bone replacement graft. Restorations and gross decay were removed from the teeth prior to extraction. Soft tissue and calculus were then removed from the teeth prior to insertion into the dentin grinder (KometaBio Smart Dentin Grinder, KometaBio, kometabio.com). The tooth was then ground into small and large particles, which were sterilized and partially demineralized according to the manufacturer's protocol.
Infra- and suprabony periodontal defects were grafted with the partially demineralized dentin, which in some cases was mixed with mineralized cancellous allograft. Any added allograft had no bioactive properties or any effect on the biological outcome of the procedure and was used solely to increase volume and potentially help stabilize the graft. After the mixture was placed in the socket and periodontal defect to obtain ideal contour, it was covered with an amnion-chorion barrier (BioXclude®, Snoasis Medical, snoasismedical.com). Primary closure was attempted but not necessarily achieved in all sites.
Radiographs of the extraction sockets and adjacent periodontal defects were taken at 1 and 3 months and beyond. Probing was not performed for 9 months after the initial surgical procedure. (Because this article is based on the study's preliminary data, no statistical analysis has been performed at the time of writing. The authors will report on this in the future after all patients have met similar timeframes for evaluation.)
Results
All teeth adjacent to the extraction sockets were maintained in a healthy periodontal state throughout the duration of the study. Dental implants were placed where appropriate and all were successfully loaded prosthetically. The gain in bone height on the adjacent treated teeth increased by 2 mm to 8 mm. This improved the prognoses on all teeth treated, none of which were extracted or had additional procedures performed.
Two representative cases are presented that demonstrate the amount of vertical periodontal augmentation obtained in the infrabony defects and above the alveolar crest in both a middle-aged and significantly older patient. Together, the two cases show that age did not limit the amount of regenerative capability of the surgical procedure and biomaterials used. Also, the second case illustrates how the results can hold up in the absence of professional care.
Case 1
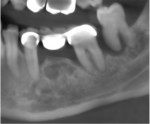
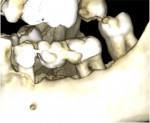
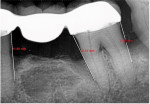
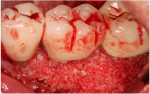
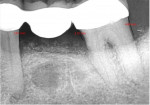
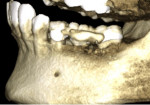
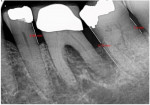
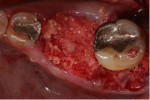
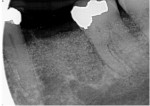
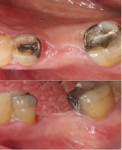
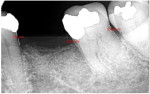
A 62-year-old male patient presented complaining of pain and mobility in the left posterior mandible. Examination noted hypereruption of the mandibular left third molar (tooth No. 17) with grade 3 mobility and a fixed prosthesis on Nos. 18 through 20 with grade 2 mobility on the distal aspect. Significant bone loss was noted on No. 17, with angular defects on the abutment teeth of bridge Nos. 18 through 20 (Figure 1). A cone-beam computed tomography (CBCT) 3D reconstruction demonstrated bone loss in this sextant (Figure 2). The depths of the bony defects were measured on the radiographs from the cementoenamel junction (CEJ) and were found to be: No. 20 distal = 9 mm, No. 18 mesial = 10 mm, and No. 18 distal = 9 mm (Figure 3). A full-mouth periodontal examination was conducted, and bleeding on probing was noted in various sites intraorally with significant probing depths in the left posterior mandible (Figure 4). Based on the data collected and the clinical findings, tooth No. 17 was deemed hopeless, and osseous grafting would be used to treat the defects associated with teeth Nos. 18 and 20, which had poor to hopeless prognoses. Tooth No. 17 would be extracted and utilized as graft material for a dentin graft following processing.
The patient presented for surgery with antibiotic premedication, and local anesthetic was administered. Tooth No. 17 was extracted and processed as an autologous dentin graft using the aforementioned dentin grinder (KometaBio Smart Dentin Grinder) following the manufacturer's protocol.11,20 Any restorations or cement can be removed intraorally or after extraction. The present authors do not use teeth that have been treated endodontically because of the potential presence of cement or other materials in the root canal chamber or accessory canals. The authors leave any residual enamel on the tooth, as most of it is dissolved by the dentin cleanser (KometaBio), which has a pH of 11. After extraction, gross decay and soft tissue are removed with hand instruments or burs. Utilization of an extracted tooth as an osseous graft source from the same patient has clinical benefits, as dentin as a graft material attracts osteoprogenitor cells and releases growth factors, including BMPs,10 producing a strong, slowly resorbing scaffold.21,22
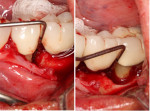
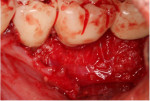
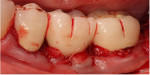
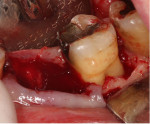
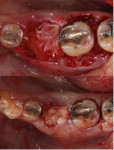
Minimal flap elevation buccally and proximally enabled access to the osseous defects associated with teeth Nos. 18 and 20 (Figure 5). The roots and defects were cleaned and debrided, then treated with EDTA (KometaBio) in preparation for graft placement. The prepared dentin graft was mixed with cancellous allograft (Maxxeus, maxxeusdental.com) and hydrated with saline. The graft mixture was placed in the defects to ideal supra-alveolar contour to regrow bone vertically (Figure 6). A dehydrated human de-epithelialized amnion-chorion membrane (BioXclude) was placed over the graft (Figure 7). The amnion-chorion membrane contains biological factors that aid with healing while promoting angiogenesis,23 reducing inflammation,24 and accelerating flap reattachment.25 The membrane has inherent antibacterial properties,26 and the tissue is non-immunogenic.27 The flap was repositioned and secured with polyglycolic acid (PGA) sutures (Figure 8). Homecare instructions were given, and the patient was dismissed.
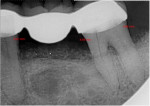
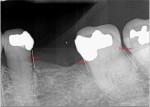
The patient was seen on regular recall to check healing. At 4 months post-surgery a periapical radiograph was taken and it was noted that the graft material was filling the defects at the mesial and distal of No. 18 and the distal of No. 20 (Figure 9). Measurements on the radiographs demonstrated significant bone fill improvement. Complete resolution of the infrabony defects and supra-alveolar growth was noted. Bone height increased on No. 20 distal by 8 mm, No. 18 mesial by 7.45 mm, and No. 18 distal by almost 6 mm.
At 8 months post-surgery the patient was seen and periodontal charting was performed (Figure 10). Probings were noted to be within normal limits, and light bleeding on probing was noted on the mesial and distal of No. 18. Mobility initially noted had reduced to grade 1. Bone distance from the crown margins were noted as follows: No. 20 distal = 3.01 mm; No. 18 mesial = 3.15 mm; and No. 18 distal = 4.69 mm. A radiograph showed stability of the previously placed graft (Figure 11). A CBCT 3D reconstruction demonstrated osseous fill of the initial defects, supra-alveolar growth, and increased radiopacity and stability of the grafts (Figure 12). The treatment improved the prognoses of teeth Nos. 18 and 20 in an 8-month timeframe from poor-to-hopeless to good on each tooth.
Case 2
An 88-year-old female patient who was a post-stroke victim with worsening periodontal issues in all posterior quadrants was seen. For the purpose of this article, this case report will focus on the mandibular left posterior; similar treatment was also performed in the maxillary left quadrant. Radiographs were taken and periodontal charting was performed. Grade 1 mobility was noted on teeth Nos. 18 and 20, while tooth No. 19 had grade 2 mobility. Radiographic bone loss was noted around all posterior teeth with complete bone loss around the distal root apex of No. 19 (Figure 13). Due to the loss of bone around the distal root of No. 19 and its grade 2 mobility it was recommended to the patient that the tooth was non-treatable and would require extraction. Further treatment would include surgical access to root plane the deep pockets around teeth Nos. 17, 18, and 20 with osseous grafting of the extraction socket and other defects using a graft created from extracted tooth No. 19.
The crown on No. 19 was sectioned and removed and the tooth was atraumatically extracted. Following minimal flap elevation to access the periodontal defects, all areas were debrided and the roots treated in a similar manner as was outlined in Case 1 (Figure 14). Extracted tooth No. 19 was processed with the aforementioned dentin grinder and partially demineralized with EDTA. The ground dentin was then mixed with cancellous allograft (Maxxeus) and formed into a leukocyte-PRF (L-PRF) block. The graft material was placed into both the extraction socket and debrided osseous defects on the adjacent teeth (Figure 15) to ideal contour and height. Amnion-chorion barrier membranes were placed over the osseous graft. Depending on the number and size of defects, either one large barrier can be cut and shaped to cover them all, or smaller barriers can be used. In this case, one 20 mm x 30 mm barrier was cut into pieces. The exposed barrier over the socket was covered with an L-PRF barrier, and resorbable sutures were placed following repositioning of the flap (Figure 16). A radiograph was taken to document the fill of the extraction socket and periodontal defects (Figure 17).
The patient presented at 8 months post-surgery and soft tissue demonstrated minimal inflammation with tissue at a stable position on the adjacent teeth and at the CEJ (Figure 18). A radiograph was taken to evaluate the graft's maturation and its blending with the adjacent host bone. Improvement of periodontal probing was noted with bone fill in all sites (Figure 19). Mobility on teeth Nos. 18 and 20 had been eliminated. A dental implant was not placed in the edentulous space due to medical contraindications.
After a long time period in which the patient was not seen due to the COVID pandemic, a radiograph was taken at 36 months post-surgery and periodontal probings were recorded. Probing depths were still in the healthy range with bone measurements of No. 18 distal at 5.16 mm, No. 18 mesial at 3.98 mm, and No. 20 distal at 4.38 mm (Figure 20). Although these readings were slightly deeper than at the 8-month recall, they demonstrated significant reduction from the initial readings.
Discussion
Traditional treatment of infrabony pockets has consisted of flap exposure of the defect with cleansing of the area to remove any soft tissue in the defect. Treatment of the exposed root surface with various compounds to achieve PDL reattachment has been recommended whether osseous grafting was performed or not depending on the depth of the defect.28 Regenerative periodontal therapy aims to re-establish the osseous anatomy and PDL reattachment that was damaged by periodontitis. Systematic reviews indicate that clinical outcomes after treatment of infrabony defects with guided tissue regeneration (GTR) were superior to open flap debridement, and the addition of osseous grafts further improved the clinical outcome of GTR.29 When osseous graft placement is part of GTR, various graft materials have been advocated and clinical success has varied between materials.30
Because of their biological properties, amnion-chorion membranes provide a reservoir of stem cells and biological scaffolds for bone regeneration. Amniotic membrane can be used for transplantation as either a temporary graft or a permanent graft used either alone or in conjunction with other surgical procedures.31 The material has been shown to be safe and well accepted by the host. Amnion-derived epithelial cells and amniotic mesenchymal stromal cells show promise for bone regeneration in animal models. These membranes are a potential alternative to commercially available membranes used for guided bone regeneration and are ideal candidates for tissue engineering strategies applied to bone healing.32 Studies have reported that the amniotic membrane can shift pro-inflammatory M1 macrophages to anti-inflammatory M2 phenotype. These M2 macrophages promote tissue repair and regeneration.33 The mesenchymal stromal/stem cell (MSC) mechanisms involved include downregulation of NF-kB signaling, which reduces pro-inflammatory cytokines like tumor necrosis factor-alpha, interleukin (IL)-1 beta, and IL-6 and induces expression of M2 markers like CD206. Human amniotic membrane extracts contain numerous growth factors and bioactive substances. These include osteogenesis-related growth factors, such as basic fibroblast growth factor, transforming growth factor beta-1, and epidermal growth factor (EGF), which differentially regulates the osteogenic differentiation of MG-63 cells.34 Additionally, amniotic MSCs reportedly also can inhibit macrophage proliferation and induce apoptosis of active macrophages via paracrine factors like PGE2 and TSG6.35
Stem cell migration is affected, as the amniotic membrane contains extracellular matrix proteins like collagen, fibronectin, and laminin, which serve as scaffolds for cell adhesion and migration. Studies demonstrate that amniotic membrane extracts promote migration of various stem cells in vitro, including bone marrow MSCs, adipose-derived MSCs, and corneal epithelial progenitor cells.19 This chemotactic activity is mediated by growth factors like hepatocyte growth factor, EGF, and VEGF in the amniotic membrane. Osteoblastic activity is increased by growth factors in the amniotic membrane, such as BMP-2, BMP-4, and BMP-7, which can stimulate osteoblastic differentiation and mineralization of bone marrow MSCs.36 Amniotic membrane extracts also upregulate expression of osteogenic genes like Runx2, osterix, and osteocalcin in MSCs. In vivo studies show amniotic membrane combined with MSCs enhances bone regeneration in critical-sized calvarial defects when compared to MSCs alone. Multiple studies support the use of amniotic membranes as an efficient alternative to current techniques for periodontal and oral soft-tissue regeneration procedures.37
Autogenous dentin graft (ADG) enables exceptional dentin and bone regeneration, with clinical, radiographic, and histologic results comparable to xenografts and autologous grafts. Overall, ADG has demonstrated biocompatibility and achieved successful bone regeneration.20 When mixed with PRF, ADG is effective in preserving post-extraction alveolar ridge dimensions.12 Autologous dentin particulate reportedly is also effective for preventing soft-tissue ingrowth, enabling graft maturation without soft-tissue interference.18 Bone replacement grafts provide demonstrable clinical improvements in periodontal bony defects compared to surgical debridement alone.38
As presented in these two cases, utilization of an amnion-chorion barrier membrane with bioactive dentin graft seems to have additive effects, with the possibility of the reforming of new bundle bone. Osteogenic and angiogenic effects of the partially demineralized dentin graft and cell recruitment and stimulation of this specific amnion-chorion barrier have been discussed in the literature. It is possible that the combination of the effects of these materials stimulates quicker graft maturation via angiogenesis and osteogenesis at early timepoints. This could enable soft tissues to migrate over the top of the healing clot more quickly than if non-bioactive materials were placed in the grafted socket and/or periodontal defect. The combination of these biological enhancements may improve not only socket fill and volume preservation buccolingually, but also supra-crestal growth of the structures needed to create and preserve bundle bone.39 This appears prominently in the postoperative radiographs in Case 2 (Figure 19 and Figure 20). Stimulation of fibroblast and PDL cell growth and migration may bring about formation of new gingival fibers perpendicular in orientation to the tooth surface. When the ADG remodels and matures, it can become bundle bone, adding to and preserving the supracrestal alveolar structure. This can lead to an improved prognosis of the treated teeth.
Socket preservation as well as fill of some portion of infrabony periodontal pockets after surgical procedures has been shown with other materials and techniques.40,41 The incorporation of bioactive materials may improve the clinical and biologic effects of healing in the infrabony components of periodontal lesions but, moreover, also seem to enable supra-alveolar bone growth. Further analysis will be performed on the patients in this study as they progress in healing to validate the current results.
Conclusion
As demonstrated in the two cases presented, and as supported by the literature, the combined use of an amnion-chorion barrier membrane with bioactive dentin graft appears to offer additive effects. Significant improvement in healing and presumed regeneration of the site being treated was shown clinically and radiographically not only in the infrabony defects, but also above the adjacent alveolar crest. Bioactive membranes stimulate host cells in the surrounding gingival tissue to accelerate site closure and healing while exerting positive effects on the underlying bone and graft material not observed to the same extent with other membranes. These benefits have been shown when treating sockets following extraction either in anticipation of implant placement following site healing or for ridge preservation. When used in repair of periodontal bone loss, reattachment of the PDL with increased bone in the crestal direction beyond the initial level of the alveolar crest (vertical periodontal augmentation) has been demonstrated.
The use of bioactive materials such as an amnion-chorion barrier and the patient's extracted tooth as graft material provides stimulus for vital bone ingrowth into the surgical site and graft conversion to host bone after site healing. Combined, an amnion-chorion membrane and autologous dentin graft material may maximize the benefits of the two individual materials, improving GTR results and the prognoses of periodontally involved teeth. Based on the literature presented and the long-lasting clinical and radiographic results shown in the cases in this study, the authors presume periodontal regeneration may have occurred. Further research is needed to determine inter-operator and inter-patient variability and what potential effect each material has on the clinical results.
DISCLOSURE
Dr. Horowitz has consulted for Snoasis Medical, Intra-Lock, and KometaBio.
ABOUT THE AUTHORS
Robert A. Horowitz, DDS
Adjunct Clinical Assistant Professor, Departments of Oral and Maxillofacial Surgery and Periodontology and Implant Dentistry, New York University College of Dentistry, New York, New York
Gregori M. Kurtzman, DDS, MAGD
Former Assistant Clinical Professor, Department of Restorative Dentistry and Endodontics, University of Maryland School of Dentistry, Baltimore, Maryland; Diplomate, International Congress of Oral Implantologists; Private Practice, Silver Spring, Maryland
REFERENCES
1. Roccuzzo A, Imber JC, Marruganti C, et al. Clinical outcomes of dental implants in patients with and without history of periodontitis: a 20-year prospective study. J Clin Periodontol. 2022;49(12):1346-1356.
2. Hwang S, Lee HM, Yun PY, Kim YK. Survival analysis of implants after surgical treatment of peri-implantitis based on bone loss severity and surgical technique: a retrospective study. BMC Oral Health. 2023;23(1):308.
3. Zhang H, Wei Y, Xu T, et al. Assessment of soft and hard tissue characteristics of ridge preservation at molar extraction sites with severe periodontitis: a randomized controlled trial. BMC Oral Health. 2022;22(1):511.
4. Zhao L, Xu T, Hu W, Chung KH. Preservation and augmentation of molar extraction sites affected by severe bone defect due to advanced periodontitis: a prospective clinical trial. Clin Implant Dent Relat Res. 2018;20(3):333-344.
5. Wei Y, Xu T, Zhao L, et al. Ridge preservation in maxillary molar extraction sites with severe periodontitis: a prospective observational clinical trial. Clin Oral Investig. 2022;26(3):2391-2399.
6. Couso-Queiruga E, Weber HA, Garaicoa-Pazmino C, et al. Influence of healing time on the outcomes of alveolar ridge preservation using a collagenated bovine bone xenograft: a randomized clinical trial. J Clin Periodontol. 2023;50(2):132-146.
7. Moghaddas O, Naddafpour N, Farhadi S, et al. Comparison of healing time and the histopathology of bone formation following tooth extraction using freeze-dried bone allograft: a randomized controlled clinical trial. J Adv Periodontol Implant Dent. 2022;14(2):69-75.
8. Rodriguez AE, Nowzari H. The long-term risks and complications of bovine-derived xenografts: a case series. J Indian Soc Periodontol. 2019;23(5):487-492.
9. Minetti E, Corbella S, Taschieri S, Canullo L. Tooth as graft material: histologic study. Clin Implant Dent Relat Res. 2022;24(4):488-496.
10. Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12(8):999-1008.
11. Kuperschlag A, Keršytė G, Kurtzman GM, Horowitz RA. Autogenous dentin grafting of osseous defects distal to mandibular second molars after extraction of impacted third molars. Compend Contin Educ Dent. 2020;41(2):76-82.
12. Pohl S, Binderman I, Tomac J. Maintenance of alveolar ridge dimensions utilizing an extracted tooth dentin particulate autograft and plateletrich fibrin: a retrospective radiographic conebeam computed tomography study. Materials (Basel). 2020;13(5):1083.
13. Smith PC, Cáceres M, Martínez C, et al. Gingival wound healing: an essential response disturbed by aging? J Dent Res. 2015;94(3):395-402.
14. Kaur J, Bathla SC. Regenerative potential of autologous platelet-rich fibrin with and without amnion membrane in the treatment of Grade-II furcation defects: a clinicoradiographic study. J Indian Soc Periodontol. 2018;22(3):235-242.
15. Sanders MC, Balaji S, Martin WB, et al. Protecting human amnion and chorion matrices during processing: performance enhancement in a diabetic mouse model and human co‐culture system. Wound Repair Regen. 2023;31(4):475-488.
16. Magatti M, Vertua E, De Munari S, et al. Human amnion favours tissue repair by inducing the M1-to-M2 switch and enhancing M2 macrophage features. J Tissue Eng Regen Med. 2017;11(10):2895-2911.
17. Melling GE, Colombo JS, Avery SJ, et al. Liposomal delivery of demineralized dentin matrix for dental tissue regeneration. Tissue Eng Part A. 2018;24(13-14):1057-1065.
18. Pohl S, Binderman I, Božić D, et al. Effectiveness of autologous tissue grafts on soft tissue ingrowth in patients following partial root extraction with socket shield: a retrospective analysis of a case series. Int J Oral Maxillofac Implants. 2021;36(2):362-370.
19. Riau AK, Beuerman RW, Lim LS, Mehta JS. Preservation, sterilization and de-epithelialization of human amniotic membrane for use in ocular surface reconstruction. Biomaterials. 2010;31(2):216-225.
20. Brunello G, Zanotti F, Scortecci G, et al. Dentin particulate for bone regeneration: an in vitro study. Int J Mol Sci. 2022;23(16):9283.
21. Campoy TB. Autologous dentin graft behavior in bone regeneration: two histologies at 5 and 10 months. Int J Periodontics Restorative Dent. 2021;41(6):835-842.
22. Um IW, Ku JK, Kim YK, et al. Histological review of demineralized dentin matrix as a carrier of rhBMP-2. Tissue Eng Part B Rev. 2020;26(3):284-293.
23. Imamura K, Hamada Y, Yoshida W, et al. Investigating the effects of dehydrated human amnion-chorion membrane on periodontal healing. Biomolecules. 2022;12(6):857.
24. Moreno SE, Massee M, Koob TJ. Dehydrated human amniotic membrane regulates tenocyte expression and angiogenesis in vitro: implications for a therapeutic treatment of tendinopathy. J Biomed Mater Res B Appl Biomater. 2022;110(4):731-742.
25. Dolivo D, Xie P, Hou C, et al. A dehydrated, aseptically-processed human amnion/chorion allograft accelerates healing in a delayed murine excisional wound model. Exp Cell Res. 2021;400(2):112512.
26. Zare-Bidaki M, Sadrinia S, Erfani S, et al. Antimicrobial properties of amniotic and chorionic membranes: a comparative study of two human fetal sacs. J Reprod Infertil. 2017;18(2):218-224.
27. Wassmer CH, Berishvili E. Immunomodulatory properties of amniotic membrane derivatives and their potential in regenerative medicine. Curr Diab Rep. 2020;20(8):31.
28. Froum S, Lemler J, Horowitz R, Davidson B. The use of enamel matrix derivative in the treatment of periodontal osseous defects: a clinical decision tree based on biologic principles of regeneration. Int J Periodontics Restorative Dent. 2001;21(5):437-449.
29. Nibali L, Koidou VP, Nieri M, et al. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: a systematic review and meta-analysis. J Clin Periodontol. 2020;47 suppl 22:320-351.
30. Brodzikowska A, Górski B, Szerszeń M, Sanz M. Efficacy of guided tissue regeneration using frozen radiation-sterilized allogenic bone graft as bone replacement graft compared with deproteinized bovine bone mineral in the treatment of periodontal intra-bony defects: randomized controlled trial. J Clin Med. 2023;12(4):1396.
31. Gupta A, Kedige SD, Jain K. Amnion and chorion membranes: potential stem cell reservoir with wide applications in periodontics. Int J Biomater. 2015:2015:274082.
32. Etchebarne M, Fricain JC, Kerdjoudj H, et al. Use of amniotic membrane and its derived products for bone regeneration: a systematic review. Front Bioeng Biotechnol. 2021;9:661332.
33. Liang G, Zhang Y. Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective. Cell Res. 2013;23(1):49-69.
34. Go YY, Kim SE, Cho GJ, et al. Differential effects of amnion and chorion membrane extracts on osteoblast-like cells due to the different growth factor composition of the extracts. PLoS One. 2017;12(8):e0182716.
35. Liu H, Jiang C, La B, et al. Human amnion-derived mesenchymal stem cells improved the reproductive function of age-related diminished ovarian reserve in mice through Ampk/FoxO3a signaling pathway. Stem Cell Res Ther. 2021;12(1):317.
36. Hao Y, Ma DH, Hwang DG, et al. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19(3):348-352.
37. Gulameabasse S, Gindraux F, Catros S, et al. Chorion and amnion/chorion membranes in oral and periodontal surgery: a systematic review. J Biomed Mater Res B Appl Biomater. 2021;109(8):1216-1229.
38. Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL, Gunsolley JC. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann Periodontol. 2003;8(1):227-265.
39. Wang RZ, Nancollas GH. Dynamics of mineral deposition in the periodontal cavity. J Dent Res. 2010;89(5):524-531.
40. Shaikh MS, Zafar MS, Alnazzawi A. Comparing nanohydroxyapatite graft and other bone grafts in the repair of periodontal infrabony lesions: a systematic review and meta-analysis. Int J Mol Sci. 2021;22(21):12021.
41. Tavelli L, Chen CY, Barootchi S, Kim DM. Efficacy of biologics for the treatment of periodontal infrabony defects: an American Academy of Periodontology best evidence systematic review and network meta‐analysis. J Periodontol. 2022;93(12):1803-1826.