Yangxi Liu, PhD; Stacey Lavender, PhD; Farid Ayad, BDS, DMD; Maha Hetata, DDS; Luis R. Mateo, MA; Carl P. Myers, PhD; Guofeng Xu, PhD; Elizabeth Gittins, BS; Yun-Po Zhang, PhD, DDS (Hon); and Bayardo García-Godoy, DMD, MSc
Abstract: Background:Dentin hypersensitivity is a global oral health concern. This in vitro study and clinical evaluation tested the efficacy of 0.454% stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) to occlude dentin and reduce dentin hypersensitivity. Methods: Human dentin specimens were treated with test and control toothpaste slurries and evaluated for dentin occlusion. In a phase III randomized controlled trial, eligible participants were randomized to SNaP toothpaste (test group), a potassium nitrate desensitizing dentifrice (positive control), or a non-desensitizing sodium monofluorophosphate dentifrice (negative control). Baseline, day 1, day 3, and day 7 dentin hypersensitivity was assessed using tactile and air blast stimuli. Mean tactile and air blast hypersensitivity scores were calculated for each treatment group. For statistical analysis, significance was set to P ≤ .05. Results: In vitro mean percent tubule occlusion for test and control samples were 86% and 35%, respectively (P < .05). One-hundred-twenty participants completed the clinical trial. After 7 days of product use, relative to the positive control and negative control groups, the test group exhibited significant reduction in tactile hypersensitivity (79.8% and 90.2%, respectively; P < .001) and reduction in air blast hypersensitivity (47.1% and 47.9%, respectively; P < .001). SNaP significantly reduced hypersensitivity pain after 1, 3, and 7 days. Conclusions: In vitro and clinical evaluation results indicate that SNaP is highly effective in coating the dentin surface, occluding exposed dentin tubules, and offering sensitivity relief from the first day of use, a benefit that continues to improve with use. Practical Implications: This multi-benefit formula reduces dentin hypersensitivity, thereby improving the daily lives of patients, promoting better oral health, and potentially helping patients avoid more serious dental problems in the future.
Dentin hypersensitivity is a global oral health concern, a significant challenge within dental practice, and it can negatively impact patients' quality of life.1-3 Dentin's innate sensitivity to stimuli is not an issue when protected by enamel and cementum; however, when dentin hypersensitivity develops due to enamel erosion, gum recession, or other factors, the tubules of dentin become exposed.2 Prevalence estimates of dentin hypersensitivity vary widely by population and healthcare setting, but a fixed-effects meta-analysis reported a population prevalence estimate of 11.5% (95% CI [11.3, 11.7]).1 Those impacted by dentin hypersensitivity report tooth pain when dentin is exposed to chemical, thermal, or other stimuli.2,4 This discomfort can limit a patient's dietary selections and negatively impact one's ability to follow recommended oral care routines.5
Consensus-based recommendations for the management of dentin hypersensitivity have warned that the condition is underdiagnosed, associated with poor health outcomes, and lacks "widespread availability of noninvasive, efficacious, and inexpensive" treatment.4 Toothpaste has been increasingly trialed with active ingredients formulated to lessen the discomfort and burden of dentin hypersensitivity.6 There are two treatment strategies to relieve hypersensitivity: agents that occlude dentin tubules, blocking the source of the discomfort, and agents that disrupt the neural pain response.2 For the latter, potassium salts are used as active agents in antisensitivity toothpaste to disrupt the pain response; however, these products can take several weeks of use for patients to feel relief.7,8
Dentin occluding agents offer an alternative approach in which hypersensitivity is addressed at its root cause as opposed to treating the symptoms.2,6 Stannous fluoride forms insoluble precipitates to occlude dentin tubules. An in vitro study found that dentin specimens treated with a stannous fluoride toothpaste had significantly more occluded dentin tubules than dentin specimens treated with a non-stannous fluoride formula containing sodium monofluorophosphate.9 When the same products were tested in a clinical trial of patients with dentin hypersensitivity, the test toothpaste provided significant improvements in tactile and air blast hypersensitivity scores compared to the negative control toothpaste at 4- and 8-week intervals.9
The following in vitro study and clinical evaluation builds on this work to test the efficacy of a 0.454% stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) to reduce dentin hypersensitivity compared to a potassium-based dentifrice and to a non-desensitizing regular 0.76% sodium monofluorophosphate dentifrice after twice-daily brushing for 1, 3, and 7 days. SNaP is formulated to offer antigingivitis, anticaries, extrinsic tooth stain removal, antisensitivity, and antibacterial benefits, as well as reduced oral malodor.10-13
Material and Methods
In Vitro Methods
Products Tested:The test group was treated with the SNaP toothpaste (Colgate-Palmolive Co., colgatepalmolive.com). The control group was treated with a negative control toothpaste containing 0.76% sodium monofluorophosphate (Colgate-Palmolive Co.).
In Vitro Assessment of Dentin Occlusion:For the in vitro assessment of dentin occlusion, confocal microscopy coupled with image analysis software (Leica Map version 7.1, Leica Microsystems, leica-microsystems.com) and visual inspection was utilized to quantify the occlusion of treated dentin specimens.
Dentin Sample Preparation:Human teeth were mounted on a saw (IsoMet® High Speed Pro, Buehler, buehler.com) and cross-sectioned into 700-µm thick slices. Cut dentin specimens were then sanded and polished on a polishing grinder (EcoMet® III, Buehler) with a polishing cloth (Buehler). Specimens were sonicated in deionized water, then etched with 1% citric acid, dried, and stored on wet tissue.
Treatment Procedure:The dentin surfaces of three specimens (per tested toothpaste) were brushed for 30 seconds, using a microbrush and toothpaste slurry. Toothpaste slurries were created using one part phosphate buffered saline (PBS) to three parts tested toothpaste. Samples were allowed to sit for 15 minutes at room temperature, placed in 10 milliliters of PBS, stirred at 125 to 130 revolutions per minute for 15 minutes, rinsed, and dried. The procedure was completed five times.
Measurement and Quantification of Occlusion:Five regions were marked on the non-sampling side of each dentin specimen. Each sample was then mounted on a glass slide with tape for imaging (DCM 3D Microscope, Leica Microsystems) before treatment (for baseline). Leica Map version 7.1 was used and coupled with an imaging and analysis program to achieve the calculation from images.14 Confocal images of each sample region were captured at 10-times magnification to identify the starting point, and acquired and reported pictures at 150-times magnification. Mean percentage of occlusion was calculated on three dentin samples for each toothpaste, with a total of 15 data points analyzed for each tested toothpaste. Percent occlusion was quantified based on the total area occupied by the open tubules of the untreated specimens against the area of any open tubules of the treated specimens. The percentage of occlusion was calculated as follows: 100 - ((R2/R1) x 100) where R1 represents the area of open tubules for untreated dentin and R2 represents the area of open tubules for treated dentin.9,14
Clinical Assessment Trial Design
The clinical assessment was a phase III randomized, single-center, double-blind, three-cell parallel-group clinical study.
Products Tested:The test group used the SNaP toothpaste (Colgate-Palmolive Co.). The first control group used a positive control desensitizing toothpaste containing 0.24% sodium fluoride and 5% potassium nitrate (GlaxoSmithKline Co., gsk.com). The second control group used a negative control toothpaste, a non-desensitizing regular 0.76% sodium monofluorophosphate toothpaste (Colgate-Palmolive Co.).
Ethics:The study (US IRB2020CP/03) was reviewed and approved by the U.S. Investigational Review Board, Inc. (U.S. IRB, Inc®), 6400 SW 72 Court, Miami, Florida 33143. All study participants signed an informed consent form.
Study Setting and Location: Healthy male and female participants were enrolled in the Costa Mesa, California, area. The recruitment period was from September 28, 2020, to September 29, 2020. The study period was from September 28, 2020, to October 21, 2020. At the clinical site, all eligible individuals were assessed by means of tactile and air blast stimuli at all study timepoints (baseline, day 1, day 3, and day 7) by the same examiner.
Participant Inclusion and Exclusion Criteria:Participants were eligible for the study if they met the following inclusion criteria: willing to sign an informed consent form; male or female adults between the ages of 18 and 70 (inclusive); in general good health as determined by the study investigators; able to participate for the full duration of the study (7 days); a minimum of two hypersensitive teeth that were anterior to the molars and demonstrated dentin exposure due to cervical erosion/abrasion or gingival recession; with qualifying dentin hypersensitivity response to tactile stimuli applied to the cervical surface as defined by a response score between 10 and 50 grams of force (Yeaple Probe, XiniX Research Inc., yeapleprobe.com); and a qualifying dentin hypersensitivity response to air blast stimuli applied for 1 second to the cervical surface (gingiva-facial third) as defined by a score of 2 or 3 on the Schiff cold air sensitivity scale.15
Participants were excluded from the study if any of the following applied: gross oral pathology, chronic disease, and/or history of allergies to any of the test products; use of any desensitizing oral care products or treatment within the past 3 months; advanced periodontal disease and/or treatment for periodontal disease within the past 12 months; hypersensitive teeth with a mobility greater than one; teeth with extensive/defective restorations or with suspected pulpitis, caries, cracked enamel, or used as abutments for removable partial dentures; current use of anticonvulsants, antihistamines, antidepressants, sedatives, tranquilizers, anti-inflammatory drugs, or daily analgesics; current participation in any oral clinical studies; self-reported pregnancy or breastfeeding; allergies to oral care products or personal care consumer products or their ingredients, or a medical condition(s) that prohibits not eating/drinking for 4 hours.
Sample Size:The sample size of 40 per group (120 total) was determined based on a standard deviation (SD) for the response measure tactile sensitivity (or air blast) of 3.34 (or 0.31), a significance level of α = 0.05, a 10% attrition rate, and an 80% level of power. The study was powered to detect a minimal statistically significant difference between the study group means of 20%.
Randomization of Treatments and Treatment Assignment:Study participants were provided with an identification number in chronological order as they were enrolled in the study. These numbers were randomly pre-assigned to a treatment group following a computer-generated randomization list. The participants enrolled in the study were randomly assigned to one of three study groups in such a way that neither the examiner nor the study participant was aware of the individual's treatment group.
Intervention:All participants were provided with their assigned toothpaste and a soft-bristled adult toothbrush and were instructed to brush for 2 minutes twice per day (once in the morning and once in the evening) for 7 days. Participants discontinued use of any other oral hygiene practices during the study period, but no restrictions were placed on dietary or smoking habits.
Clinical Scoring Procedures:Study participants were instructed to refrain from any oral hygiene procedure and/or chewing gum for 8 hours, and from eating and/or drinking for 4 hours prior to each scheduled visit (baseline, day 1, day 3, and day 7) to the clinical site. Participants were screened by the dental examiner.
Tactile Hypersensitivity Assessment:Participants' tactile sensitivity was assessed by use of the Model 200A Electronic Force-Sensing Probe developed by Yeaple Research of Pittsford, New York. The application of this probe for dentin sensitivity testing utilizing a #19 explorer tip at a preset force measured in grams was employed.
Teeth were evaluated for tactile hypersensitivity in the following manner16,17:(1) Participants were instructed to respond at the point when they first experienced discomfort. (2) The explorer tip of the probe was applied to the buccal surface of each sensitive tooth at the cementoenamel junction. (3) The explorer tip was stroked perpendicular to the tooth beginning at a preset force of 10 grams and increasing by 10-gram increments until the participant experienced discomfort or 50 grams of force was applied. If there was no indication of discomfort upon application of 50 grams of force, the tooth was deemed nonsensitive to tactile stimulation and ineligible for inclusion in the study.
Air Blast Hypersensitivity Assessment:Air blast evaluations were conducted approximately 5 minutes after tactile evaluation. Teeth that were identified as sensitive and which demonstrated abrasion, erosion, and/or gingival recession were evaluated in the following manner: (1) The sensitive tooth was isolated from the adjacent teeth (mesial and distal) by the placement of the examiner's fingers over the adjacent teeth. (2) Air was delivered/ejected from a standard dental unit air syringe at 60 PSI (±5 PSI) and 70°F (±3°F). (3) The air was directed at the exposed buccal surface of the sensitive tooth for 1 second from a distance of approximately 1 centimeter. The Schiff cold sensitivity scale was used to assess the participant's response to this stimulus.15 Sensitivity was scored as follows: 0 = tooth/participant does not respond to air stimulus; 1 = tooth/participant responds to air stimulus but does not request discontinuation of stimulus; 2 = tooth/participant responds to air stimulus and requests discontinuation or moves from stimulus; 3 = tooth/participant responds to air stimulus, considers stimulus to be painful, and requests discontinuation of the stimulus.
Monitoring/Reporting of Adverse Events
All clinical complaints, symptoms, or signs that met the adverse event definition were recorded on a case report form. Adverse events were assessed by the investigator or designee for severity, relationship to the study product, possible etiologies, and whether the event met the criteria as a serious adverse event.
Statistical Methods: In Vitro Study
Data analysis was conducted by using statistical software (Minitab version 18.1, Minitab, minitab.com) with a student's t-test comparing the mean percentage of occlusion for each of the toothpastes. Differences between treatments were statistically significant if the P value was less than or equal to .05.
Statistical Methods: Clinical Assessment
Data analysis was performed on tactile and air blast hypersensitivity assessments by using statistical software (Minitab version 18.1). Comparisons of the study treatment group demographics were analyzed using a chi-square test to assess gender and an independent student's t-test for age. Comparison of the treatment groups with respect to baseline tactile and air bast hypersensitivity, and baseline compared to follow-up, were analyzed with an independent student's t-test. The between-treatment comparisons with respect to baseline-adjusted tactile and air blast hypersensitivity at the follow-up examinations were analyzed using an analysis of covariance (ANCOVA) model. Differences within and between treatments were statistically significant if P value was less than or equal to .05.
Results
In Vitro Results
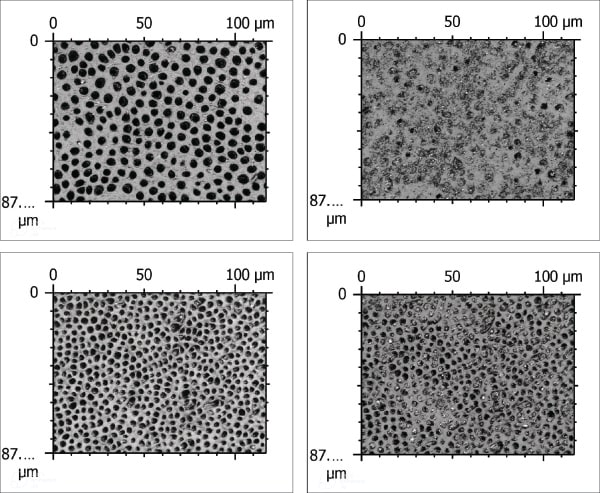
Confocal microscopy images (representative images are shown in Figure 1 through Figure 4) were acquired by means of confocal microscopy of dentin specimens treated with test and negative control toothpastes. Confocal images of the test specimens before and after five treatments (Figure 1 and Figure 2) as well as the control specimens before and after five treatments (Figure 3 and Figure 4) were collected. The visual inspection aligned with the quantified mean percent occlusion for both treatment groups. Dentin specimens treated with the test toothpaste had 86% occlusion, whereas the negative control toothpaste had 35% occlusion. The difference between these two products was significantly different at a 95% confidence level according to results with a two-sample t-test (P < .05).
Clinical Trial Results
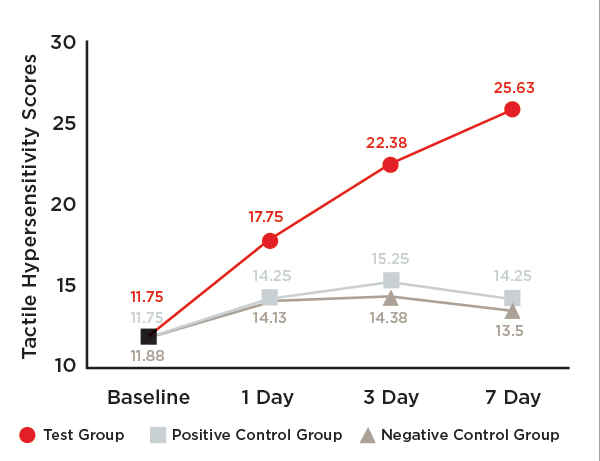
One-hundred-twenty (120) participants complied with the protocol and completed the clinical study (Figure 5). The trial stopped at the end of the study period. Mean age and ranges are reported in Table 1. No statistically significant differences were indicated among the three groups at baseline with respect to either tactile hypersensitivity (P = .980) or air blast hypersensitivity (P = .982). No subgroup analyses were performed. Unadjusted tactile and air blast scores are reported in Table 2, Figure 6, and Figure 7.
Tactile Hypersensitivity:The test toothpaste, SNaP, provided statistically significant improvements in dentin hypersensitivity after 1, 3, and 7 days (Figure 6).
After 1 day of product use, the percent improvements in tactile hypersensitivity from baseline were 51.1% (P < .001) for the test group, 21.3% (P = .001) for the positive control group, and 18.9% (P < .001) for the negative control group (Table 3). Relative to participants in the positive control and negative control groups, those in the test group exhibited statistically significant improvements of 24.5% (P < .001) and 26.4% (P < .001), respectively, in tactile hypersensitivity scores (Table 3).
After 3 days of product use, the percent improvements in tactile hypersensitivity from baseline were 90.5% (P < .001) for the test group, 29.8% (P < .001) for the positive control group, and 21.0% (P < .001) for the negative control group (Table 4). The test group also exhibited statistically significant improvements when compared to the positive control and negative control groups: 46.6% (P < .001) and 56.1% (P < .001), respectively (Table 4).
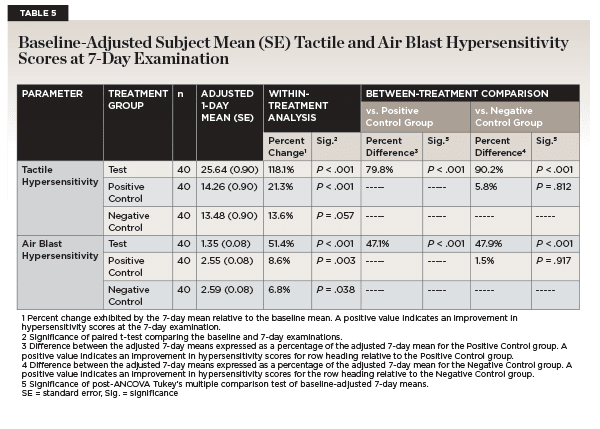
After 7 days of product use, the percent improvements in tactile hypersensitivity from baseline were 118.1% (P < .001) for the test group, 21.3% (P < .001) for the positive control group, and 13.6% (P = .057) for the negative control group (Table 5). Relative to participants in the positive control and negative control groups, those in the test group exhibited statistically significant improvement in tactile hypersensitivity (79.8% and 90.2%, respectively; P < .001) (Table 5).
Air Blast Hypersensitivity:The test toothpaste, SNaP, provided statistically significant improvements in air blast hypersensitivity after 1, 3, and 7 days (Figure 7).
After 1 day of product use, the percent reductions in air blast hypersensitivity from baseline were 24.8% (P < .001) for the test group, 5.7% (P = .003) for the positive control group, and 7.2% (P = .002) for the negative control group (Table 3). Relative to participants in the positive control and negative control groups, those in the test group exhibited statistically significant improvements of 20.2% (P < .001) and 19.0% (P < .001), respectively, in air blast sensitivity scores after 1 day of product use (Table 3).
After 3 days of product use, the percent reductions in air blast hypersensitivity from baseline were 41.0% (P < .001) for the test group, 12.9% (P < .001) for the positive control group, and 10.4% (P < .001) for the negative control group (Table 4). Relative to participants in the positive control and negative control groups, those in the test group exhibited statistically significant reductions of 32.2% (P < .001) and 34.1% (P < .001), respectively, in air blast hypersensitivity scores after 3 days of product use (Table 4).
After 7 days of product use, the percent reductions in air blast hypersensitivity from baseline were 51.4% (P < .001) for the test group, 8.6% (P = .003) for the positive control group, and 6.8% (P = .038) for the negative control group (Table 5). Finally, after 7 days of product use, relative to participants in the positive control and negative control groups, those in the test group exhibited statistically significant reductions of 47.1% (P < .001) and 47.9% (P < .001), respectively, in air blast hypersensitivity scores (Table 5).
Adverse Events:No adverse events were observed by the investigator or reported by the study participants.
Discussion
Confocal microscopy confirmed that the SNaP toothpaste was highly effective in coating the dentin surface and occluding dentin tubules. Image analysis of the confocal images helped verify that 86% of the tubules were occluded after in vitro treatment with SNaP. The negative control toothpaste, resulting in 35% occlusion after treatment, was less effective. Visual inspection of confocal images helped confirm that most of the tubules were occluded fully after treatment with the stannous fluoride toothpaste, whereas most of the dentin tubules treated with the negative control toothpaste remained open. In vitro results alone are promising but do not prove efficacy in a real-world setting.
However, in addition to the dentin occlusion confirmed in the in vitro results, SNaP provided hypersensitivity relief in a clinical evaluation. The randomized trial was well controlled and utilized double-blinding methodology. SNaP provided a statistically significant improvement in hypersensitivity after 1, 3, and 7 days of product use as compared to a commercially available potassium-based toothpaste and a non-desensitizing regular fluoride toothpaste containing 0.76% sodium monofluorophosphate. The clinical results highlight the significant and faster sensitivity reductions (1 day) provided by stannous fluoride toothpaste occlusion technology compared to a potassium nitrate desensitizing toothpaste, which did not show significant reductions from the negative control even after 7 days. For both tactile and air blast hypersensitivity scores, the percentage difference for the SNaP toothpaste test group increased as a function of time. While the sample was sufficiently sized to show clinically meaningful results, it was a single-site study and results may not be applicable to all patient groups.
Dentin hypersensitivity is known to interfere with recommended oral care routines. Cyclically, poor oral care routine then contributes to periodontal diseases associated with root exposure and root caries, which then increases the risk of incidence or worsening of dentin hypersensitivity.5,18,19 A desensitizing, tubule-occluding toothpaste that reduces hypersensitivity in less than a week interrupts this cycle and, in alignment with consensus-based recommendations,4 provides low-cost and widely available treatment for dentin hypersensitivity patients.
Conclusions
In vitro results indicate that SNaP toothpaste was highly effective in coating the dentin surface and occluding exposed dentin tubules, which is the root cause of dentin hypersensitivity. SNaP provided a statistically significant reduction in dentin hypersensitivity after 1, 3, and 7 days of product use as compared to a commercially available desensitizing potassium-based toothpaste and a regular fluoride toothpaste containing 0.76% sodium monofluorophosphate. This multi-benefit SNaP toothpaste holds promise to improve oral care routines and, ultimately, the oral health and quality of life for patients suffering from dentin hypersensitivity.
ACKNOWLEDGMENTS
Technical writing was provided by Cynthia Drake Morrow, PhD, MA, and Jennifer Wisdom, PhD, MPH, ABPP. The author contributions were as follows: YL: conceptualization, methodology, formal analysis, visualization; SL: conceptualization, supervision; FA and MH: investigation, methodology, resources; LM: formal analysis; CM, GX, and EG: resources; YZ: conceptualization, funding acquisition; BG: conceptualization, supervision. All authors contributed to writing, review, and editing.
DISCLOSURES
This clinical trial was supported by funding from the Colgate- Palmolive Company. ClinicalTrials.gov: NCT06244290. The study was reviewed and approved by the U.S. Investigational Review Board, Inc. (U.S. IRB, Inc®), 6400 SW 72 Court, Miami, Florida 33143. The authors YL, SL, CM, GX, EG, YZ, and BG are employees of Colgate-Palmolive Co. CM and GX have patents #US10918580B2 and #US11723846B2 issued to Colgate-Palmolive Co.
DATA AVAILABILITY
The documents containing the results of the research herein described are confidential. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
About the Authors
Yangxi Liu, PhD
Senior Research Scientist Research and Development (R&D), Colgate-Palmolive Co., Piscataway, New Jersey
Stacey Lavender, PhD
Senior Director R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Farid Ayad, DDS, MSc
Principal Investigator, FAR Oral and Systemic Health Consulting, Inc., Costa Mesa, California
Maha Hetata, DDS
Co-Investigator and Examiner, FAR Oral and Systemic Health Consulting, Inc., Costa Mesa, California
Luis R. Mateo, MA
President, LRM Statistical Consulting LLC, West Orange, New Jersey
Carl P. Myers, PhD
Director R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Guofeng Xu, PhD
Senior Director R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Elizabeth Gittins, BS
Senior Manager Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
Yun-Po Zhang, PhD, DDS (Hon)
Senior Vice President and Distinguished Fellow, Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
Bayardo García-Godoy, DMD, MSc
Director Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
References
1. Zeola LF, Soares PV, Cunha-Cruz J. Prevalence of dentin hypersensitivity: systematic review and meta-analysis. J Dent.2019;81:1-6.
2. Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent. 2013;14(3):136-145.
3. West NX. Dentine hypersensitivity: preventive and therapeutic approaches to treatment. Periodontol 2000. 2008;48:31-41.
4. Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc.2003;69(4):221-226.
5. Chu CH, Lo ECM. Dentin hypersensitivity: a review. Hong Kong Dent J. 2010;7:15-22.
6. Martins CC, Riva JJ, Firmino RT, Schünemann HJ. Formulations of desensitizing toothpastes for dentin hypersensitivity: a scoping review. J Appl Oral Sci. 2022;30:e20210410.
7. Orchardson R, Gillam DG. The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain. 2000;14(1):9-19.
8. Poulsen S, Errboe M, Mevil YL, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev.2006;3:CD001476.
9. Hines D, Xu S, Stranick M, et al. Effect of a stannous fluoride toothpaste on dentinal hypersensitivity: in vitro and clinical evaluation. J Am Dent Assoc. 2019;150(4S):S47-s59.
10. Lee S, Li Y, Mateo LR, et al. A 6-month randomized controlled trial to measure the efficacy of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dental plaque and gingivitis. Compend Contin Educ Dent.2024;45 suppl 3:21-29.
11. Chakraborty B, Seriwatanachai D, Triratana T, et al. Antibacterial effects of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP): in vitro study and randomized controlled trial. Compend Contin Educ Dent. 2024;45 suppl 3:12-20.
12. Elias-Boneta AR, Mateo LR, D'Ambrogio R, et al. Efficacy of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) in extrinsic tooth stain removal: a randomized controlled trial. Compend Contin Educ Dent.2024;45 suppl 3:46-52.
13. Cabelly A, Bankova M, Darling J, et al. Stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) reduces oral malodor: a randomized clinical study. Compend Contin Educ Dent.2024;45 suppl 3:40-45.
14. Sullivan R, inventor; Colgate-Palmolive Co., assignee. Image processing of dentin tubules. US20160232666A1. August 11, 2016. https://patents.google.com/patent/US20160232666A1/en. Accessed September 24, 2024.
15. Schiff T, Dotson M, Cohen S, et al. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: a twelve-week clinical study. J Clin Dent. 1994;5 spec no:87-92.
16. Clark GE, Troullos ES. Designing hypersensitivity clinical studies. Dent Clin North Am. 1990;34(3):531-544.
17. Gillam DG, Bulman JS, Jackson RJ, Newman HN. Efficacy of a potassium nitrate mouthwash in alleviating cervical dentine sensitivity (CDS). J Clin Periodontol. 1996;23(11):993-997.
18. von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29 suppl 3:173-177.
19. Chen W, Zhu T, Zhang D. The prevalence and common risk indicators of root caries and oral health service utilization pattern among adults, a cross-sectional study. PeerJ. 2023;11:e16458.