Antibacterial Effects of a Novel Stannous Fluoride Toothpaste Stabilized With Nitrate and Phosphates (SNaP): In Vitro Study and Randomized Controlled Trial
Brinta Chakraborty, PhD; Dutmanee Seriwatanachai, PhD; Terdphong Triratana, DDS; Luis R. Mateo, MA; Robert D’Ambrogio, BS; Guofeng Xu, PhD; Maria Ryan, DDS, PhD; and Yun-Po Zhang, PhD, DDS (Hon)
Abstract: Background: Stannous fluoride has long been an effective antibacterial, anticaries, antisensitivity, and antigingivitis addition to toothpaste formulas. However, in the past its chemical properties in aqueous solution have made it difficult to stabilize with desirable results. The recent development of a novel formulation of 0.454% stannous fluoride stabilized with nitrate and phosphates (SNaP) has resulted in prolonged therapeutic effect without compromising product experience and esthetics. Methods: Dentifrice antibacterial performance in vitro was determined through bacterial bioenergetics measured via rate of oxygen consumption and extracellular acidification in real-time comparing the SNaP toothpaste, a stannous fluoride positive control toothpaste, a non-antibacterial negative control toothpaste, and no treatment. Also, a single-center, randomized, controlled, double-blinded, clinical investigation of 98 subjects was performed to analyze dentifrice antibacterial performance in vivo following twice daily treatment with SNaP toothpaste (n = 48) and non-antibacterial control toothpaste (n = 50). Oral microenvironments, including plaque, tongue, cheek, gum, and saliva, of study participants 12 hours post-brushing were analyzed for bacterial load at baseline, 2 weeks, and 4 weeks. Results: In vitro treatment of biofilms with SNaP toothpaste resulted in significant suppression of bacterial respiration and glycolysis compared to a positive control, negative control, and no treatment. In the clinical trial, treatment with SNaP toothpaste showed significantly lower bacterial load in all oral microenvironments 12 hours post-brushing after 2 weeks (all: P < .01) and 4 weeks (all: P < .05) compared to non-antibacterial negative control toothpaste. Compared to baseline, SNaP toothpaste significantly reduced bacteria from tongue (P = .007) and saliva (P < .001) at week 2, and from all microenvironments by week 4 (all: P ≤ .001). Conclusions: SNaP toothpaste provided significantly greater and more sustained antibacterial effects than other tested toothpastes. Stannous fluoride, when stabilized in the SNaP formulation, effectively inhibited bacterial respiration and glycolysis in saliva-derived in vitro biofilms. The specific stabilization strategy used in SNaP toothpaste is critical for the antibacterial performance of stannous fluoride, as this formulation was more effective at reducing bacterial metabolic activity than a toothpaste containing the same amount of stannous fluoride stabilized with gluconate. The clinical study supports the in vitro findings by showing that the regular use of SNaP toothpaste leads to a significant and prolonged reduction in viable bacterial counts of five oral microenvironments. Practical Implications:The highly stabilized stannous ion in SNaP toothpaste confers potent, sustained antibacterial activity that can contribute to improved oral hygiene and potentially reduce the risk of tooth decay, early gum disease, calculus, and halitosis, which have been linked to oral bacteria.
Humans are host to vast communities of microbiota that, when maintained in balance, are critical to human immunological, metabolic, and physiological function. Multiple distinct microbial habitats exist just within the mouth.1 These oral microenvironments, such as the teeth, tongue, cheek, gum, and saliva, support the growth of highly heterogeneous and significantly different bacterial assemblies.2 Bacteria in community naturally form complex networks, called biofilms, which attach to these oral surfaces; but the combination of the nutrient-rich environment of the mouth and nature of modern-day dietary consumption can result in the accumulation of oral biofilms into dental plaques.3Plaque control plays an integral role in preventing microbial imbalances that lead to dental caries, gingivitis, and periodontitis.4 Thus, toothbrushing with fluoridated toothpaste and interdental prophylaxis are recommended to fight caries and reduce plaque accumulation. However, 42.2% of adults in the United States over 30 years old have mild, moderate, or severe periodontitis.5 Evidence suggests that the large percentage of adults with poor oral health is the result of poor technique or irregularity of brushing and the type of dentifrice used, which could be improved by the inclusion of a toothpaste with antimicrobial properties.6
Stannous fluoride (SnF2) has long set a historical precedent as a highly effective antibacterial, anticaries, and antigingivitis addition to toothpaste formulas.7-9 The antibacterial performance of SnF2 outperforms other fluoride-based therapeutics and is dependent on maintaining Sn2+ ions, which interfere with bacterial metabolic function, slowing growth, reducing cellular respiration, and preventing production of bacterial acid through glycolysis.8,10 However, in aqueous conditions SnF2 can readily hydrolyze to form therapeutically inactive Sn4+ ions.10-13 Previous approaches to improve on Sn2+ ion stability have included the removal of water to prevent hydrolysis, which is costly, presents processing difficulties, and compromises product desirability, and the addition of more Sn2+ salts, which compromises taste and can increase tooth staining.14 An additional approach involves the use of complexation agents, such as gluconate, lactate, and polyphosphates, to chelate stannous. Clinical efficacy of SnF2 toothpastes relies on the prolonged stability of the 2+ oxidation state of stannous.
The recent development of a proprietary SnF2 formula combines the properties of phosphates and nitrate ions to solubilize and stabilize Sn2+ ions, prolonging the therapeutic efficacy of both F- and Sn2+ without compromising flavor, mouth feel, or whitening capabilities.14-16 In vitro studies reveal that this formulation significantly suppresses the growth of Streptococcus mutans and Porphyromonas gingivalis, while approximately 90% of Sn2+ ions remain in solution after 2 weeks.14
The objective of the study herein was twofold. The first portion was an in vitro study measuring the prolonged antimicrobial impact of toothpaste containing 0.454% stannous fluoride stabilized with nitrate and phosphates (SNaP) on cultured salivary biofilms compared to a commercially available SnF2 toothpaste formulation and a non-antibacterial, non-stannous, fluoride toothpaste. The second part was a clinical study to evaluate the antibacterial effects of toothpaste containing SNaP compared to a non-antibacterial negative control sodium monofluorophosphate toothpaste in vivo.
Materials and Methods
In Vitro Biofilm Investigation
Dentifrice Treatment:The test treatment was toothpaste containing 0.454% stannous fluoride stabilized with nitrate and phosphates (SNaP). The negative control in this portion of the study was a non-antibacterial toothpaste containing 0.24% sodium fluoride (NaF) and 5% potassium nitrate (GlaxoSmithKline Co., gsk.com). The positive control treatment was toothpaste containing 0.454% stannous fluoride stabilized with sodium gluconate (SnF2 + SG) (Procter & Gamble, pg.com).
Biofilm Culture:Biofilms derived from saliva of healthy volunteers were cultured vertically on hydroxyapatite discs for approximately 48 hours at 37°C, 5% carbon dioxide aerobic conditions in McBain medium containing 5 µg/ml hemin and 1 µg/ml vitamin K.17 The media were replaced twice daily at approximately 12-hour intervals. The biofilm from one disc was transferred to a 24-well plate containing 1 ml of McBain medium. The biofilm was further dispersed by vigorous pipetting and transferred to an adjacent well. Toothpaste treatments were applied to 15 µl of the dispersed biofilm suspension, which contained approximately 106 cells per suspension.
Bacterial OCR and ECAR Measurements:Bacterial metabolic function18,19 was measured using a Seahorse XFe24 cell analyzer (Agilent, agilent.com). Bioenergetics following treatment with the toothpastes were quantified in real time by measuring oxygen consumption rate (OCR; pmol/min) and extracellular acidification rate (ECAR; mpH/min). Briefly, bacteria were seeded and immobilized in microplates using Cell-Tak™ (Corning, ecatalog.corning.com), and bacterial metabolism in the presence or absence of treatments was measured for up to 200 minutes. Toothpaste slurries were prepared by mixing one part of toothpaste with eight parts of water, after which the slurry was spun down to remove solid material, and 10 µl of toothpaste supernatant was used in each experimental well. The experiments were carried out in 375 µl of McBain media at 37°C with intermittent shaking. The area under the curve was calculated for each treatment using GraphPad-Prism version 9.0 for Windows (GraphPad Software, graphpad.com). Data are representative of four independent experiments performed at least in triplicates. A two-sided t-test was used to assess statistical significance.
Clinical Investigation
Dentifrice Treatment:The test treatment was SNaP toothpaste containing 0.454% stannous fluoride. The negative control treatment in the clinical portion of the study was a non-antibacterial toothpaste containing 0.76% sodium monofluorophosphate (MFP) (Colgate-Palmolive Co., colgatepalmolive.com).
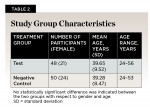
Study Design and Participants:This double-blind, single-center, two-arm, parallel, randomized, controlled clinical investigation (NCT06353165) was approved by the Institutional Review Board of Mahidol University. One-hundred healthy female and male adults (50 participants per group) between the ages of 18 and 70 years old were recruited March 1-3, 2023, from the greater Bangkok, Thailand, area. The study took place between March 8, 2023, and April 12, 2023. The number of participants needed for this study was calculated as previously described.20,21 Qualifying participants were randomized into two treatment groups. Randomization was performed using the random number calculator of the GraphPad QuickCalcs website: graphpad.com/quickcalcs (accessed March 2023). The test group received non-identifiable SNaP toothpaste, and the negative control group received MFP toothpaste. Original toothpaste tubes were covered with coded white labels.
Inclusion and Exclusion Criteria: Inclusion criteria were as follows: a minimum of 20 natural teeth with facial and lingual scorable surfaces; a baseline whole-mouth score of dental plaque of 1.5 or more22,23 and gingivitis index of 1.0 or more24,25; no allergies to oral hygiene formulations; a willingness to comply with all study procedures and clinical examination schedules. Exclusion criteria were as follows: a history of active or severe periodontal disease and loose teeth; gross dental caries; severe generalized abrasion of dental cervix and/or enamel; large tooth fracture or temporary restoration (based on visual examinations); fixed or removable orthodontic appliance or removable partial dentures; dental prophylaxis or treatments within the preceding month or during the study period; use of phenolic flavored products (eg, mint flavored candies and chewing gum) during the study period; difficulty complying with study procedures and examinations (eg, excessive gagging during oral assessment or inability to refrain from oral hygiene for 12 hours prior to scheduled visit); medical treatments including antibiotic, anti-inflammatory, or anticoagulant therapy during the preceding month or during the study period; history of medical conditions requiring prophylactic antibiotic treatment prior to invasive dental procedures; history of significant adverse effects following use of oral hygiene products such as toothpastes and mouthrinses; allergy to personal care/consumer products or their ingredients; history of alcoholism or recreational drug use (including habit-forming products), diabetes, hepatic or renal disease, inflammatory conditions, or serious transmittable diseases (eg, HIV); participation in another clinical study or test panel involving oral hygiene formulations within the preceding month; having a scheduled medical procedure during the study period; self-reported pregnancy or lactation during the study period.
Study Protocol:Participants were given a soft-bristled toothbrush and instructed to brush twice daily for 2 minutes using a full ribbon of toothpaste and to refrain from using other oral hygiene products during the study. Non-antibacterial negative control toothpaste, MFP, was provided for a 7-day washout period.
Twelve hours after the last day of the washout period, participants returned to the study site for the baseline evaluation (day 0), where investigators examined the oral cavity and asked safety questions. Oral microenvironment samples were collected in the form of oral rinse saliva, supra-gingival plaque, tongue scrapings, buccal mucosa scrapings, and gum scrapings. Participants received prelabeled anonymized toothpastes containing either SNaP or MFP. Participants returned for examination, questioning, and sample collection at 14 days + 12 hours for the week 2 (day 15) and 28 days + 12 hours for the week 4 (day 29) timepoints.
Sample Collection and Microbial Procedures:Saliva was collected by providing participants with 15 ml of sterile saline to rinse their mouths for 30 seconds and expectorate into a prelabeled sterile tube. Supragingival plaque was randomly collected from buccal surfaces of the upper right or left quadrant (teeth Nos. 2 through 8 or teeth Nos. 9 through 15) using a sterile Columbia 13/14 scaler, pooled, and placed in a tube containing 1 ml of sterile phosphate buffered saline (PBS). Tongue, cheek, and gum surface samples were collected using the edge of a sterile wooden disposable tongue depressor and comprised five scrapes per site from a defined area randomly chosen. The inside of the right or left cheek or an entire arch of the upper or lower jaw was scraped. Each tongue depressor was then placed into a tube containing 3 ml of sterile PBS. Oral microenvironment samples were vortexed for 30 seconds to shake loose the biofilms and sonicated for 30 seconds before serial dilution in PBS and plating on agar enriched with 5% sheep blood as previously described.26
Statistical Analyses
The gender and age compositions of the two treatment groups were compared using chi-squared analysis and analysis of variance (ANOVA), respectively. Raw data were logarithmically transformed (base 10) prior to statistical analysis. Baseline values between the two treatment groups were compared using ANOVA. Within-treatment baseline versus week 2 and week 4 comparisons were performed using paired t-tests. Between-treatment comparisons of baseline-adjusted week 2 and week 4 oral sample values were performed using analysis of covariance (ANCOVA) with baseline value as covariate. All statistical tests of hypotheses were two-sided and used a significance level of α = 0.05. Minitab version 18.1 (Minitab, minitab.com) was used to perform the analyses. The percent change in bacterial counts was calculated by using (1-10^ΔLog CFU) x 100%.
Results
In Vitro Biofilm Investigation
To compare the antibacterial performance of the novel SNaP formulation to other commercially available dentifrices in vitro, biofilm cultures derived from saliva of healthy volunteers were processed with the SNaP test toothpaste, a market brand stannous toothpaste (SnF2 + SG), a non-antibacterial, non-SnF2 negative control toothpaste (NaF), and no treatment. Antibacterial performance was measured by monitoring bacterial metabolic function for bacterial respiration via OCR and glycolysis via ECAR. Over a 200-minute observation period, SNaP toothpaste treatment exhibited notable suppression of OCR and ECAR compared to both the market brand stannous formula and negative control (Figure 1 and Figure 2). SnF2 + SG showed OCR suppression for the first 50 minutes but bacterial respiration proceeded to increase over time for the remaining trial, while SNaP toothpaste suppressed OCR for the entire 200 minutes (Figure 1). Glycolytic activity was suppressed by both SnF2 + SG and SNaP toothpaste treatments compared to the negative control; however, SNaP continued to keep ECAR at near zero levels throughout the entire experiment (Figure 2).
To further assess the significance of SNaP toothpaste suppression of bacterial respiration and glycolysis, area under the curve was measured and statistical significance was analyzed (Table 1). Both the SNaP and SnF2 + SG toothpaste treatments were significantly lower than NaF negative control and no treatment in OCR and ECAR (P < .05). SNaP and SnF2 + SG treatments resulted in 97.8% and 51.1% less oxygen consumption than the NaF negative control treatment, respectively. Notably, the level of oxygen consumption following SNaP toothpaste treatment was 95.6% less than the SnF2 + SG dentifrice (P < .05). SNaP and SnF2 + SG treatments also resulted in a 93.3% and 80.5% lower rate of glycolysis than treatment with the NaF negative control, respectively. Again, the level of glycolysis following SNaP toothpaste treatment was 65.7% less than the SnF2 + SG positive control (P < .05). There was no statistically significant difference between NaF negative control treatment and no treatment in both OCR and ECAR.
Clinical Investigation
Study Design: One-hundred participants were recruited and 50 were randomly allocated into either an antibacterial test group or a non-antibacterial negative control group (Figure 3). No statistically significant difference was indicated between the two treatment groups with respect to gender (P = .673) and age (P = .841) (Table 2). Of the 100 study participants, 98 completed the study (Figure 3). Two participants from the test group did not attend all study visits for reasons unrelated to adverse events and were excluded from analysis.
The per protocol participants brushed twice daily for 2 minutes using a full ribbon of toothpaste and refrained from use of other oral hygiene products during the study. Following a 7-day washout period, oral microenvironment samples were collected at baseline, 2 weeks, and 4 weeks in the form of oral rinse saliva, supragingival plaque, tongue scrapings, buccal mucosa (cheek) scrapings, and gum scrapings. Statistical analyses were performed on oral microenvironment bacterial load log10 (CFU/mL) assessments.
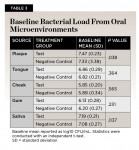
Baseline Analysis:No significant difference was observed between the two treatment groups at baseline with respect to tongue (P = .364), cheek (P = .565), and gum (P = .231) bacterial load (Table 3). In saliva, the negative control group (7.08 ± 0.27 log10 CFU/mL) exhibited significantly (P = .037) less bacteria than the test group (7.19 ± 0.21 log10 CFU/mL). Plaque bacteria was also significantly (P = .038) lower in the negative control group (7.33 ± 3.38 log10 CFU/mL) than in the test group (7.47 ± 0.23 log10 CFU/mL).
Week 2 Analysis:By week 2, there was a significant difference between treatment group bacterial counts for all oral microenvironments (Table 4). The test group exhibited significantly lower bacterial loads than the negative control group for plaque (P < .001), tongue (P < .001), cheek (P < .001), gum (P = .003), and saliva (P < .001).
Compared to baseline measurements, the test group had a significant mean decrease in log10 CFU/mL bacterial load from tongue samples (P = .007) and saliva samples (P < .001). Meanwhile, the negative control group had a significant mean increase of log10 CFU/mL bacteria from plaque (P = .004). All other baseline-adjusted mean bacteria counts were not significantly different at 2 weeks compared to baseline (Table 4).
Week 4 Analysis:At week 4, there continued to be a significant difference between treatment group bacterial counts for all oral microenvironments (Table 5). The test group exhibited significantly lower bacterial loads than the negative control group for plaque (P < .001), tongue (P = .022), cheek (P < .001), gum (P < .001), and saliva (P = .001).
Four weeks after baseline measurements, the test group showed significant decrease in log10 CFU/mL bacteria from all oral microenvironments compared to baseline (Table 5). The negative control group only exhibited a significant decrease in log10 CFU/mL bacteria from tongue (P = .013) and saliva samples (P = .038).
Adverse Events:No staining of the participants' teeth was reported in either treatment group. No adverse events were noted during the examinations of the oral cavity at any visit, nor were any reported by the participants.
Discussion
Treatment with the novel SnF2 dentifrice formula, SNaP, resulted in significant and clinically relevant suppression of bacterial metabolism and growth across in vitro and in vivo studies. Despite equal amounts of the active ingredient SnF2, SNaP consistently outperformed comparator toothpaste, SnF2 + SG, in a prolonged manner in vitro. These results suggest that the nitrate/phosphates stannous stabilization approach used in SNaP toothpaste maintains bioavailable stannous to a greater extent than traditional stannous chelator systems like sodium gluconate. SNaP's antibacterial properties were also evident clinically, where it significantly reduced bacterial load in all oral microenvironments tested compared to commercially available non-antibacterial fluoride toothpaste 12 hours post-brushing after both 2 weeks and 4 weeks of continuous use.
SNaP toothpaste treatment of salivary biofilm cultures resulted in long-lasting and significant suppression of bacterial respiration and glycolytic activity in comparison to treatment with the same amount of SnF2 stabilized with sodium gluconate. Studying bacterial respiration and glycolytic activity via OCR and ECAR is crucial for understanding how SNaP toothpaste impacts oral bacterial metabolism. By measuring OCR, researchers can determine how effectively the toothpaste inhibits the aerobic respiration of harmful oral bacteria, while ECAR measurements reveal its effects on glycolytic and fermentation processes. These insights are essential for evaluating the efficacy of a product in real-time, ensuring it effectively disrupts the metabolic activities of pathogens responsible for dental plaque and cavities. These results are supported by previous single-species bacterial inhibition studies that found that SNaP significantly inhibited the growth of common oral disease-associated bacteria, S mutans and P gingivalis, compared to SnF2 without nitrate.14 In aqueous solution, approximately 90% of Sn2+ ions remain in solution after 2 weeks when stabilized with phosphates and nitrate ions compared to <10% of Sn2+ that remain when SnF2 is not stabilized with phosphates and/or nitrate ions.14 This evidence indicates that the prolonged antibacterial effects of SNaP are due to the novel formula allowing for extended stability and bioavailability of Sn2+.
The antibacterial properties of SNaP toothpaste seen in vitro were observed in vivo as well. After 4 weeks of continuous use, SNaP toothpaste significantly reduced bacterial count in saliva, supragingival plaque, tongue scrapings, buccal mucosa (cheek) scrapings, and gum scrapings compared to baseline levels in clinical trial groups. Notably, despite lower baseline levels of bacteria in plaque and saliva in the negative control group at baseline, bacteria from plaque and saliva increased to be higher than the SNaP toothpaste-treated test group by week 2. Use of SNaP toothpaste resulted in significantly lower amounts of bacteria 12 hours after brushing for all oral microenvironments tested compared to MFP toothpaste. These results are supported by evidence that 0.454% SnF2 is able to significantly reduce biofilm formation, cell adhesion, and quorum sensing on tooth surfaces and oral devices compared to MFP.27
While previous studies have shown that brushing with a SnF2 dentifrice reduces the buildup of dental calculus, plaque, gingivitis, tooth stain, and malodor,28 the extended bioavailability observed from the SNaP formulation may allow for a greater long-term therapeutic effect. The sustained antibacterial impact on both hard- and soft-tissue surfaces may also prevent soft-tissue sites from acting as microbial reservoirs that reseed teeth with bacteria following prophylaxis.6 The results of this can be seen in other SNaP clinical trials yielding prolonged malodor reduction along with significant reductions in plaque, gingivitis, and dentin hypersensitivity.16,29,30
Further research on the SNaP formula could elucidate the exact mechanisms of action driving its prolonged antibacterial efficacy. Recent research leveraging next-generation sequencing has shown that certain oral pathogens appear to be more impacted than others by stannous fluoride27,31; future studies could help uncover the comprehensive profiles of microbial communities affected by SNaP, offering insights into shifts in bacterial diversity and the relative abundance of oral microorganisms induced by this oral care ingredient. Identifying metabolic changes and biochemical pathways impacted by SNaP treatment and understanding how it modulates bacterial activity at the molecular level could help design formulation strategies to enhance its efficacy and provide long-term oral health outcomes.
Toothbrushing with fluoridated toothpaste and interdental prophylaxis are recommended to maintain oral health. However, periodontitis remains a clear issue for a large percentage of adults who may not have optimal brushing practices.5,6 The incorporation of stable antibacterial ingredients like stannous fluoride in SNaP toothpaste is important to prevent bacterial-driven oral conditions.
Conclusion
SNaP toothpaste provides superior, sustained antibacterial effects that can help prevent the growth of harmful bacteria without any reported staining or other unpleasant consequences. The fact that these antibacterial effects were not limited to the enamel surfaces but also proven in four additional areas of the mouth supports the use of SNaP toothpaste to maintain whole-mouth health.
ACKNOWLEDGMENTS
The authors thank the study participants for contributing their time and Meghan A. Berryman, PhD, for assistance in writing the manuscript. The author contributions were as follows: BC: conceptualization, methodology, formal analysis, visualization; DS and TT: investigation, methodology, resources; LM: formal analysis; RD and GX: resources; MR: conceptualization, funding acquisition; YZ: conceptualization, funding acquisition, supervision. All authors contributed to writing, review, and editing.
DISCLOSURES
This clinical trial was supported by funding from the Colgate-Palmolive Company. ClinicalTrials.gov: NCT06353165. The study was reviewed and approved by the Institutional Review Board of Mahidol University, Bangkok, Thailand. The authors BC, RD, GX, MR, and YZ are employees of Colgate-Palmolive Co. GX has patents #US10918580B2 and #US11723846B2 issued to Colgate-Palmolive Co.
DATA AVAILABILITY
The documents containing the results of the research herein described are confidential. The authors confirm that the data supporting the findings of this study are available within the article and/or its supplementary materials.
About the Authors
Brinta Chakraborty, PhD
Senior Research Scientist Research and Development (R&D), Colgate-Palmolive Co., Piscataway, New Jersey
Dutmanee Seriwatanachai, PhD
Associate Professor, Department of Oral Biology, Faculty of Dentistry, Mahidol University, Bangkok, Thailand
Terdphong Triratana, DDS
Associate Professor, Department of Oral Biology, Faculty of Dentistry, Mahidol University, Bangkok, Thailand
Luis R. Mateo, MA
President, LRM Statistical Consulting LLC, West Orange, New Jersey
Robert D'Ambrogio, BS
Senior Principal Scientist R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Guofeng Xu, PhD
Senior Director R&D, Colgate-Palmolive Co., Piscataway, New Jersey
Maria Ryan, DDS, PhD
Executive Vice President, Clinical Research, Knowledge Management and Scientific Communications, Colgate-Palmolive Co., Piscataway, New Jersey
Yun-Po Zhang, PhD, DDS (Hon)
Senior Vice President and Distinguished Fellow, Clinical Research, Colgate-Palmolive Co., Piscataway, New Jersey
References
1. Kilian M, Chapple ILC, Hannig M, et al. The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221(10):657-666.
2. Xu X, He J, Xue J, et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 2015;17(3):699-710.
3. Rosier BT, De Jager M, Zaura E, Krom BP. Historical and contemporary hypotheses on the development of oral diseases: are we there yet? Front Cell Infect Microbiol. 2014;4:92.
4. Paqué PN, Karygianni L, Kneubuehler J, et al. Microbial approaches for the assessment of toothpaste efficacy against oral species: a method comparison. Microbiologyopen. 2022;11(2):e1271.
5. Eke PI, Thornton-Evans GO, Wei L, et al. Periodontitis in US adults: National Health and Nutrition Examination Survey 2009-2014. J Am Dent Assoc. 2018;149(7):576-588.e6.
6. Haraszthy VI, Raylae CC, Sreenivasan PK. Antimicrobial effects of a stannous fluoride toothpaste in distinct oral microenvironments. J Am Dent Assoc.2019;150(4S):S14-S24.
7. Nevitt GA, Witter DH, Bowman WD. Topical applications of sodium fluoride and stannous fluoride. Public Health Rep. 1958;73(9):847-850.
8. Myers CP, Pappas I, Makwana E, et al. Solving the problem with stannous fluoride: formulation, stabilization, and antimicrobial action. J Am Dent Assoc. 2019;150(4S):S5-S13.
9. White DJ. A "return" to stannous fluoride dentifrices. J Clin Dent. 1995;6 spec no:29-36.
10. Tinanoff N. Review of the antimicrobial action of stannous fluoride. J Clin Dent. 1990;2(1):22-27.
11. Pettine M, Millero FJ, Macchi G. Hydrolysis of tin(II) in aqueous solutions. Anal Chem. 1981;53(7):1039-1043.
12. Food Drug Administration. Oral health care drug products for over-the-counter human use; antigingivitis/antiplaque drug products; establishment of a monograph. Proposed Rule. Fed Regist. 2003;68(103):31937-32322.
13. Desmau M, Alsina MA, Gaillard JF. XAS study of Sn speciation in toothpaste. J Anal At Spectrom. 2021;36(2):407-415.
14. Zhang S, Govindaraju GV, Cheng CY, et al. Oxidative stability of chelated Sn(II)(aq) at neutral pH: the critical role of NO3− ions. Sci Adv. 2024;10(40). doi: 10.1126/sciadv.adq0839.
15. Elias-Boneta AR, Mateo LR, D'Ambrogio R, et al. Efficacy of a novel stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) in extrinsic tooth stain removal: a randomized controlled trial. Compend Contin Educ Dent. 2024;45 suppl 3:46-52.
16. Cabelly A, Bankova M, Darling J, et al. Stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) reduces oral malodor: a randomized clinical study. Compend Contin Educ Dent. 2024;45 suppl 3:40-45.
17. McBain AJ, Sissons C, Ledder RG, et al. Development and characterization of a simple perfused oral microcosm. J Appl Microbiol.2005;98(3):624-634.
18. Lamprecht DA, Finin PM, Rahman MA, et al. Turning the respiratory flexibility of mycobacterium tuberculosis against itself. Nat Commun.2016;7:12393.
19. Saini V, Cumming BM, Guidry L, et al. Ergothioneine maintains redox and bioenergetic homeostasis essential for drug susceptibility and virulence of mycobacterium tuberculosis. Cell Rep. 2016;14(3):572-585.
20. Hu D, Zhang YP, Petrone M, et al. Clinical effectiveness of a triclosan/copolymer/sodium-fluoride dentifrice in controlling oral malodor: a three-week clinical trial. Compend Contin Educ Dent. 2003;24(9 suppl):34-41.
21. Hu D, Li X, Liu H, et al. Evaluation of a stabilized stannous fluoride dentifrice on dental plaque and gingivitis in a randomized controlled trial with 6-month follow-up. J Am Dent Assoc. 2019;150(4S):S32-S37.
22. Quigley GA, Hein JW. Comparative cleansing efficiency of manual and power brushing. J Am Dent Assoc. 1962;65:26-29.
23. Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41(1):41-43.
24. Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533-551.
25. Talbott K, Mandel ID, Chilton NW. Reduction of baseline gingivitis scores with repeated prophylaxes. J Prev Dent. 1977;4(6):28-29.
26. Haraszthy VI, Sreenivasan PK. Microbiological and clinical effects of an oral hygiene regimen. Contemp Clin Trials Commun. 2017;8:85-89.
27. Gumber HK, Louyakis AS, Sarma T, et al. Effect of a stannous fluoride dentifrice on biofilm composition, gene expression and biomechanical properties. Microorganisms. 2022;10(9):1691.
28. Johannsen A, Emilson CG, Johannsen G, et al. Effects of stabilized stannous fluoride dentifrice on dental calculus, dental plaque, gingivitis, halitosis and stain: a systematic review. Heliyon. 2019;5(12):e02850.
29. Lee S, Li Y, Mateo LR, et al. A 6-month randomized controlled trial to measure the efficacy of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dental plaque and gingivitis. Compend Contin Educ Dent. 2024;45 suppl 3:21-29.
30. Liu Y, Lavender S, Ayad F, et al. Effect of a stannous fluoride toothpaste stabilized with nitrate and phosphates (SNaP) on dentin hypersensitivity: in vitro study and randomized controlled trial. Compend Contin Educ Dent.2024;45 suppl 3:30-39.
31. Fine N, Barbour A, Kaura K, et al. Effects of a stabilized stannous fluoride dentifrice on clinical, immunomodulatory, and microbial outcomes in a human experimental gingivitis model. J Periodontol. 2024;95(5):421-431.