Antibiotic Stewardship in the Management of Endodontic Infections
Reducing the incidence of adverse events and the burden of antibiotic resistance
Brooke Blicher, DMD | Rebekah Lucier Pryles, DMD | Jarshen Lin, DDS
Although antibiotics are lifesaving tools used to manage serious infections, their use is associated with morbidity and mortality on an individual patient and population level. Allergies, antibiotic resistance, and disruption of normal intestinal flora that results in overgrowth of pathogenic organisms are unintended sequelae of antibiotic use with potentially dire consequences. To reduce the incidence of adverse events, antibiotic stewardship requires that practitioners align their prescribing habits with the best available evidence and avoid writing inappropriate prescriptions. The dental literature clearly defines both indications and contraindications for antibiotic use in the management of endodontic infections. Following these guidelines ensures that any antibiotics prescribed are both necessary and effective. This article outlines the indications and contraindications for antibiotic use and includes an evidence-based review of appropriate drugs and dosages to effectively manage infections of endodontic origin.
Antibiotic stewardship necessitates that these lifesaving drugs be used only in appropriate cases and in an appropriate manner. The extensive use of antibiotics in dentistry and medicine is a major source of antibiotic resistance, which has rendered once-treatable infections lethal.1 Drug- and multidrug-resistant infections are common worldwide, and the development of pathogens that are resistant to all known antibiotics will likely occur. The World Health Organization repeatedly issues warnings regarding antibiotic-resistant superbugs and recommends prioritizing research to focus on the most dangerous pathogens.2 However, the pharmaceutical industry has been slow to respond because it faces financial pressures to focus research on other, more lucrative arenas.
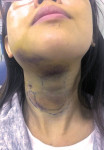
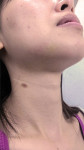
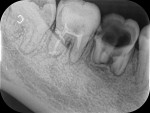
Drug-resistant infections are encountered with increasing frequency in dentistry, and both the undertreatment of and delayed treatment of dental infections can result in potentially serious conditions. Failure to manage dental infections in a timely fashion can result in rapid spread of cellulitis through fascial planes, which may become life-threatening.3 Once that occurs, infections often require hospitalization for management with IV antibiotics in the intensive care unit. Hospitalization brings undue morbidity and cost to the management of cases that might have been managed more efficiently by the administration of oral antibiotics just days prior (Figure 1 through Figure 3).
Given these findings, the dental community must respond to issues of antibiotic misuse within the profession. Antibiotic stewardship for endodontic infections requires adherence to evidence-based guidelines. Both the American Association of Endodontists and the European Society of Endodontology have published clear indications and contraindications for the use of antibiotics in the management of endodontic infections (Table 1).4,5
Indications for antibiotics include infections that show signs of systemic involvement characterized by fever, malaise, lymphadenopathy, or trismus as well as infections in medically compromised patients. Infections that show signs of rapid progression, such as onset within 24 hours; cellulitis, or an otherwise spreading infection; and cases of osteomyelitis also should receive systemic antibiotic therapy.5 In addition, cases involving traumatic dental injuries in which a completely avulsed tooth is replanted or in which soft-tissue trauma otherwise requires sutures or debridement may require administration of systemic antibiotics.5,6 Referral to an oral surgeon or other medical provider should be considered for cases in which the intravenous administration of antibiotics might be required.5
Contraindications for antibiotic use in managing endodontic pathology also exist. Chronic infections or acute infections that remain localized do not require antibiotics.4,5 Cases of pulpal disease, such as symptomatic irreversible pulpitis, in which pain is present but there are no other signs and symptoms of infection do not require antibiotics. Pulpal necrosis and periradicular disease, including symptomatic and asymptomatic apical periodontitis, presenting with widening of the periodontal ligament space or periapical pathology, with or without pain on percussion and biting, also do not require antibiotic prescription. Furthermore, as long as there are no signs of systemic involvement, cases of chronic apical abscess for which a sinus tract and periapical radiolucency is present as well as cases of acute apical abscess with localized fluctuant swelling do not require the use of antibiotics.4,5 Lastly, systemic antibiotics are not indicated in cases involving other traumatic injuries, including fractures and luxation injuries.6
Clear indications for antibiotic use warrant selection of an appropriate drug at the appropriate dose. Prescriptions are most often written based on clinical empiricism rather than culture and sensitivity testing. Endodontic infections are polymicrobial, containing both gram-positive and gram-negative varieties of facultative anaerobes and strict anaerobes.7 Consequently, antibiotics prescribed in dental settings must be indicated for bacteria with these characteristics. To reduce the incidence of antibiotic resistance as well as prevent alterations in the normal intestinal flora, the most suitable drug with the narrowest spectrum should be selected in each case. Antibiotic use can disrupt the normal intestinal flora, allowing for the overgrowth of certain bacteria, particularly Clostridium difficile,which can result in severe gastroenteritis and an increased risk of mortality.
To prevent a treatable infection from rapidly progressing into one that is life-threatening, the selected dose must be within the therapeutic window. Although variability in patient physiology and the particular bacteriologic makeup of the infection can make accurate dosing problematic, dosages must be high enough to reach the minimum inhibitory concentration of the drug when given at intervals of three to four times the serum half-life of the drug. In cases of acute dental infection, loading dosages of twice the maintenance dose are advised to achieve therapeutic antibiotic blood levels sooner.8-10 However, if resistance is encountered, culture and sensitivity testing can be utilized to determine an appropriate substitute.4
Penicillin V, the first-line antibiotic in treating endodontic infections, is a narrow-spectrum drug with bacteriocidal action that inhibits cell wall synthesis in gram-negative, facultative anaerobes and strict anaerobes.8,9,11 Its relatively short half-life necessitates a dosage of 500 mg every 6 hours.10,12 Patients with compromised immune systems or who have demonstrated previous penicillin resistance warrant selection of a drug with a broader spectrum, greater half-life, and higher oral absorption—all of which can be offered by amoxicillin.4,9 Amoxicillin is administered at 500 mg every 8 hours.4,12
When penicillin or amoxicillin are ineffective in reducing the signs and symptoms of infection within 48 hours to 72 hours, metronidazole (500 mg every 6 hours to 8 hours) can be added as an adjunct to these drugs.4,9 Metronidazole is bacteriocidal and inhibits DNA synthesis. Because it is effective only against strict anaerobes, it must be used in conjunction with other antibiotics.11 It has a significant interaction with alcohol; therefore, patients must be counseled about avoiding any alcohol or alcohol containing products (eg, mouth rinses) until at least 48 hours after completion of a course of metronidazole.
When resistance is encountered due to beta-lacatamase producing bacterial species, amoxicillin combined with clavulanic acid (ie, Augmentin®) can provide an alternative to combining metronidazole with amoxicillin or penicillin.4,9 Augmentin has been shown to be extremely effective against endodontic infections.11 However, it can directly contribute to further antibiotic resistance and has a higher profile of associated side effects, including increased risk of infection by C. difficile, so its use should be limited. It is most conveniently administered at a dose of 875 mg every 12 hours.12
For patients who are allergic to penicillin, clindamycin should be the first-line drug.4,8,9 Clindamycin is a bacteriostatic agent that blocks protein synthesis. It is effective against most gram-positive aerobes as well as both gram-positive and gram-negative anaerobes and facultative anaerobes.9 It should be administered at a dose of 300 mg every 6 hours to 8 hours, depending on the severity of the infection.4,9,12 Clindamycin is especially effective against endodontic infections, presumably due to its high availability in bony tissue.13 However, clindamycin's particularly broad spectrum also predisposes patients to the risk of infection by C. difficile, so its use should be limited to those cases in which narrower spectrum drugs are contraindicated.
When drugs in the penicillin family or clindamycin cannot be used, clarithromycin (250 mg every 12 hours) or azithromycin (500 mg followed by 250 mg once daily) may be prescribed.4,9 Both drugs are in the macrolide family and are known to be effective against a variety of aerobic and anaerobic gram-positive and gram-negative bacteria. They are bacteriostatic and inhibit protein synthesis. Table 2 presents the antibiotics used to treat endodontic infections.4,5,9,12 For an algorithm to aid in the selection of the most appropriate antibiotic, see “Algorithm for Appropriate Antibiotic Selection to Treat Endodontic Infection.”4,5,8,9
There is no official consensus on the appropriate duration for a course of antibiotics beyond that point when the signs and symptoms of infection are resolved. However, it is important to recognize that endodontic infections may fail to completely resolve until the source of infection is treated definitively, either by extraction of the offending tooth, completion of endodontic therapy, pulpectomy, and/or possibly incision and drainage, when warranted. In clinical practice, most prescriptions are intended to be taken for approximately 5 days to 7 days with instructions for patients to continue the course for a few days beyond symptom resolution.4 Current recommendations, which are based on the principles of antibiotic stewardship, indicate shorter prescriptions of just 3 days combined with close monitoring during the antibiotic course and for 2 days to 3 days after to ensure that symptoms do not recur.5,8
Clinicians must, above all else, do no harm. Antibiotic stewardship falls within this mandate. Although antibiotics provide useful adjunctive treatment for dental infections, they are not inherently curative. Consequently, when managing endodontic infections, the focus must remain on definitive treatment. However, when antibiotics become necessary, adherence to evidence-based guidelines not only protects individual patients, but also society as a whole.
About the Authors
Brooke Blicher, DMD
Upper Valley Endodontics, PC
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Clinical Instructor
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Rebekah Lucier Pryles, DMD
Upper Valley Endodontics, PC
White River Junction, Vermont
Assistant Clinical Professor
Department of Endodontics
Tufts University School of Dental Medicine
Boston, Massachusetts
Jarshen Lin, DDS
Director of Predoctoral Endodontics
Department of Restorative Dentistry and Biomaterials Science
Harvard School of Dental Medicine
Boston, Massachusetts
Clinical Associate
Department of Oral and Maxillofacial Surgery
Massachusetts General Hospital
Boston, Massachusetts
References
1. American Dental Association Council on Scientific Affairs. Combating antibiotic resistance. J Am Dent Assoc. 2004;135(4):484-487.
2. McNeil DG. Deadly ‘Superbugs' Post a Huge Threat, W.H.O. Says. New York Times. February 27, 2017. www.nytimes.com. Accessed November 2, 2017.
3. Siqueira JF, Rôças IN. Microbiology and Treatment of Endodontic Infections. In: Hargreaves K, Berman LH. Cohen's Pathways of the Pulp. 10th ed. St Louis, MO: Mosby; 2011:559-600.
4. American Association of Endodontics. Antibiotics and the Treatment of Dental Infections. Endodontics: Colleagues for Excellence. Summer 2006.
5. American Association of Endodontists. AAE position statement: AAE guidance on the use of systemic antibiotics in endodontics. J Endod. 43(9):1409-1413.
6. The Recommended Guidelines of the American Association of Endodontists for the Treatment of Traumatic Dental Injuries Page. https://www.nxtbook.com/nxtbooks/aae/traumaguidelines/index.php. Updated 2013. Accessed November 2, 2017.
7. Siqueira JF, Rôças IN. Present status and future directions in endodontic microbiology. Endodontic Topics. 2014;30(1):3-22.
8. American Association of Endodontics. Use and Abuse of Antibiotics. Endodontics: Colleagues for Excellence. Winter 2012.
9. Segura-Egea JJ, Gould K, Sen BH, et al. Antibiotics in Endodontics: a review. Int Endod J.2016. doi:10.1111/iej.12741.
10. Pallasch TJ. Pharmacokinetic principles of antimicrobial therapy. Periodontol 2000. 1996;10:5-11.
11. Baumgartner JC, Xia T. Antibiotic susceptibility of bacteria associated with endodontic abscesses. J Endod. 2003;29(1):44-47.
12. Ganda KM. Dentist's guide to medical conditions and complications. Ames, IA: Wiley-Blackwell; 2008.
13. Nicholas P, Meyers BR, Levy RN, et al. Concentration of clindamycin in human bone. Antimicrob Agents Chemother. 1975;8(2):220-221.