Vital Pulp Therapy: Managing Deep Caries in the Permanent Dentition
Jason Juang, DMD; Crystal Song, DMD; Rebekah Lucier Pryles, DMD; and Brooke Blicher, DMD
Abstract: The adoption of vital pulp therapy (VPT) is a significant advancement in preserving the longevity of vital mature pulp. VPT represents a potential alternative approach to nonsurgical root canal therapy in which compromised pulp is treated such that it maintains its vitality and function. With the introduction of novel bioceramic materials, including calcium silicate cements and mineral trioxide aggregate, the prognosis for VPT in mature permanent teeth has greatly increased, and as a result, adaptation of VPT in these teeth has garnered considerable support. This article reviews evidence-based guidance for case selection and procedural methods associated with the adoption of VPT in mature permanent teeth.
Pulpal injury or exposure due to caries presents a pathway for microbial ingress into the pulp tissue that serves as the etiology for endodontic pathology if left untreated. Nonsurgical root canal therapy (NSRCT) currently is the most common treatment modality for endodontic pathology, namely pulpitis or pulp necrosis, in the permanent dentition. This treatment removes the exposed, inflamed, or necrotic pulp tissue and seals the canal with a synthetic biomaterial. In certain cases, vital pulp therapy (VPT) may represent an alternative approach that addresses the compromised pulp while allowing it to maintain its vitality and function.1 VPT encompasses a continuum of procedures spanning from pulp capping (placement of a protective material over the exposed pulp) to pulpotomy (removal of the coronal pulp).2 Whether or not VPT may be utilized as a treatment modality is determined by the degree of pulpal inflammation.1
Historically, VPT was reserved for the treatment of immature permanent teeth to facilitate completion of root apex formation, known as apexogenesis.2 In the context of mature permanent dentition, VPT was not traditionally viewed as a viable treatment option, because mature pulp was perceived to lack the dynamic healing capacity that afforded younger pulp a high success rate in regenerative endodontics.1 With the introduction of novel bioceramic materials, including various calcium silicate cements (CSCs) and mineral trioxide aggregate (MTA), the prognosis for VPT in mature permanent teeth has increased significantly and is now reported as having success rates as high as 93.2%.3 As a result, adaptation of VPT in mature teeth has garnered support.1
The adoption of VPT is a significant advancement in preserving the longevity of vital mature pulp. Because any dental procedure is associated with risk, including NSRCT, the ability to delay more aggressive care based on the adoption of more conservative procedures is beneficial. For example, NSRCT can compromise tooth strength due to canal enlargement and dentin loss.4 Recurrent disease following NSRCT can necessitate more aggressive interventions such as extraction. VPT offers a proactive approach, allowing clinicians to delay this treatment progression and maintain tooth integrity.
This article reviews evidence-based guidance for case selection and procedural methods associated with the adoption of VPT in mature permanent teeth.
Diagnosis and Case Selection
Proper case selection for VPT plays a crucial role in achieving a favorable long-term prognosis. As the name suggests, a prerequisite of VPT is the presence of vital pulp tissue. Therefore, case selection should begin with establishing an accurate endodontic diagnosis for the tooth in question with confirmation, via intratreatment visual assessment, of vital, bleeding tissues.1 Prior to treatment, pulp sensibility testing should be utilized to determine the pulpal status in order to assess the appropriateness of VPT. This involves both thermal testing (using cold or heat) and electric pulp testing (EPT). The estimation of pulpal health is based on the intensity and duration of the tooth's sensory response.5 While thermal testing, especially cold testing, is generally preferred over EPT due to its superior diagnostic capability in cases of pulpitis, patients with teeth affected by sclerosis from age or trauma may exhibit reduced responsiveness to thermal testing.5 In such cases, EPT serves as a viable alternative to assess for any indications of pulp necrosis. With any diagnostic test, the value of a control cannot be underscored enough. Control testing of adjacent teeth is essential for the establishment of an accurate and complete diagnosis.
Endodontic diagnoses associated with the presence of vital pulp tissue include those with a normal pulp, reversible pulpitis, symptomatic irreversible pulpitis, or asymptomatic irreversible pulpitis.5,6 Teeth with normal pulp exhibit a typical thermal response consistent with testing in control teeth. In contrast, teeth diagnosed with reversible pulpitis display an exaggerated but non-lingering response to thermal stimuli.5 On the other hand, teeth with symptomatic irreversible pulpitis exhibit a heightened and lingering response to cold activation due to C-fiber activation and inflammation-induced dentin hypersensitivity.7 The designation of asymptomatic irreversible pulpitis is made both clinically and radiographically, with the presence of a carious pulp exposure and either normal thermal test results or heightened but non-lingering responses to thermal stimuli.5 Recent histological studies challenge the conventional belief that teeth diagnosed with "irreversible pulpitis" have reached a stage beyond recovery, necessitating NSRCT. Instead, these studies reveal that pulpitis falls on a spectrum ranging from initial to mild, moderate, and severe.8 Success of VPT hinges on where the pulp stands in this continuum of histopathological involvement.1 It is important to recognize that pulpal diagnosis is a general guideline to help filter out non-viable cases. Even with the presence of vital pulp, not all cases will respond positively to VPT.
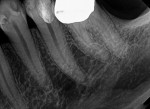
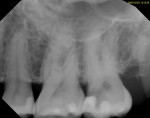
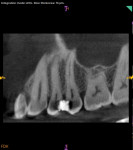
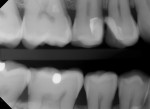
The prognosis for VPT is tied to several clinical factors. The size and location of the carious exposure are notable considerations. A more favorable prognosis is often associated with pulp exposures smaller than 2.5 mm (Figure 1 through Figure 5).9 Conversely, the prognosis tends to be less promising in cases of class V exposures, primarily due to the increased number of dentin tubules in cervical areas compared to proximal surfaces, leading to a heightened degree of inflammation.
The selection of VPT in mature permanent teeth is predicated on the presence of vital pulp tissue, as established by the pulpal diagnosis. Several contraindications, aside from the presence of necrotic pulp tissue, exist with respect to VPT, including the spread of pulpal disease to periapical tissues.1 Teeth diagnosed with either symptomatic or asymptomatic apical periodontitis are associated with less predictable outcomes following VPT.
Treatment Protocol
Informed Consent
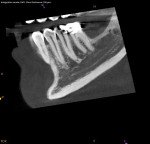
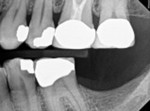
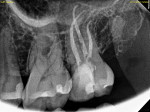
Any case being considered for VPT must undergo informed consent. This should include discussion with the patient that VPT success is not guaranteed. As mentioned previously, diagnosis is only a general guideline that does not provide introspection on the true histological state of the pulp, which is often best assessed after complete caries excavation.8 Therefore, it is also imperative to discuss the possibility of multiple complications arising, including an intratreatment decision to complete NSRCT and the potential for further pulpal disease warranting NSRCT. The necessity for these progressive treatments should then prompt discussion of additional restorative needs, including the potential need for full-coverage restorations following NSRCT on posterior teeth (Figure 6 and Figure 7).10
Local Anesthesia
Profound clinical anesthesia must be achieved prior to any work to ensure patient comfort, particularly because teeth with pulpitis may exhibit resistance to local anesthetics.11 Generally, two carpules of 2% lidocaine in 1:100,000 epinephrine through buccal infiltration should achieve proper anesthesia in maxillary teeth. In the mandible, in most cases two carpules of 2% lidocaine in 1:100,000 epinephrine or 3% mepivacaine plain via inferior alveolar nerve block in conjunction with buccal infiltration of 4% articaine in 1:100,000 epinephrine will suffice.11
Dental Dam
Use of dental dam isolation is the standard of care for endodontic treatment. It minimizes the risk of contamination of the root canal system by bacteria in the oral cavity. Asepsis is required for successful VPT, and dental dam isolation is considered the only acceptable isolation methodology for VPT treatment.12
Materials
Success rates for direct pulp caps in mature permanent teeth vary greatly and depend on several factors, such as who performed the study (endodontic specialists versus undergraduate students), the type of material placed in proximity to the pulp, whether temporary versus definitive filling materials were placed over the capping material, the type of isolation used, and magnification quality.13
The single greatest predictor of prognosis, however, is material selection. Historically, the recommended material for direct pulp caps was calcium hydroxide. Bioceramic materials, namely CSCs and MTA, have become the new recommended choices for VPT given their significantly greater predictability.3 When CSCs or MTA are used in conjunction with proper protocol, success rates for VPT in mature permanent teeth range from 85% to 100%.2,14The improved outcomes are associated with the ability of these materials to induce growth factor release, thus enhancing pulpal cell proliferation, differentiation, and migration.2,3,15
Caries Excavation
The American Association of Endodontists' position statement on VPT urges that "complete caries removal is essential to eliminate infected tissues… residual caries compromises necessary observations of pulpal inflammation levels and areas of potential necrosis."1 Although other authors advocate for selective caries removal procedures, this approach contradicts the protocols described here and is outside the scope of this article.16
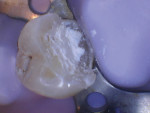
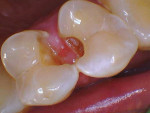
If no pulp exposure is found after complete caries removal, the tooth may be conventionally restored. Calcium hydroxide can be placed as a base material in the deepest areas of the preparation, as the placement of bioceramics is not indicated unless pulp exposure is noted.17 If a pulp exposure is noted, hemostasis must be achieved, because the success of VPT hinges on the extent of pulpal inflammation and the ability to achieve hemostasis. Generally, VPT can be completed, provided that hemostasis is achieved within 5 to 10 minutes under applied pressure with a cotton pellet soaked in 2.5% sodium hypochlorite (NaOCl).15 The use of NaOCl in pulpal lavage plays a pivotal role in improving pulpal survival rates and minimizing contamination. In comparison to pulps treated with other solutions, such as saline, with a 1-year success rate of 55%, pulps treated with NaOCl show higher success rates of up to 89%.18 Teeth exhibiting limited bleeding and ready hemostasis are good candidates for a direct pulp cap using a bioceramic material at a thickness ranging from 0.5 mm to 1 mm (Figure 8 through Figure 10).
When hemostasis is not readily achieved, a partial pulpotomy may be considered; this involves the removal of inflamed tissue while preserving the remaining apical portion of the coronal pulp tissue. If bleeding persists even after removal of inflamed tissue, complete removal of coronal pulp (ie, a full pulpotomy) is necessary.19 Bioceramic materials again represent the material of choice for placement over pulp tissues following partial or full pulpotomy.19 If hemostasis is not achieved even after a full pulpotomy, NSRCT should be the clinician's next option, as the tooth is no longer a suitable candidate for VPT. In all cases, teeth should receive a definitive restoration appropriate to the tooth structure remaining and treatment completed.
Follow-up
Follow-up appointments are essential to evaluate for any symptoms and provide radiographic monitoring for signs of apical pathology or resorption after VPT. The quality of the coronal seal should be assessed, as prognosis of VPT is significantly reduced in cases with bacterial microleakage. As a general guideline, follow-up visits should begin and be more frequent at every 3 to 6 months and may later be extended to every 6 to 12 months or as needed.20 It is important to communicate to patients that despite undergoing VPT, there are inherent risks of potential failure over time. Therefore, ongoing informed consent discussions similar to those conducted before initiating treatment should highlight the possibility of the need for additional future interventions as follow-up evaluations progress.10
Conclusion
Bioceramic materials have revolutionized VPT, offering a highly effective and conservative solution for mature permanent teeth affected by pulp injury or exposure.2 When encountering vital teeth exhibiting symptoms of pulpal disease due to carious exposure, clinicians should consider VPT as a potential treatment modality. Adopting the simple workflow of obtaining hemostasis with sodium hypochlorite and applying bioceramic materials under a dental dam can delay the need for root canal treatment.12,15 While VPT may not always serve as a definitive solution, its substantial advantage lies in enabling the deferral of more invasive treatments. VPT is especially beneficial for younger patients, as it allows for an extended time period of tooth preservation by postponing the cycle of treatment and retreatment, which can involve having to redo root canal treatments and crowns due to failure over time. By integrating VPT into clinical practice, clinicians can promote long-term tooth retention.
About the Authors
Jason Juang, DMD
Harvard School of Dental Medicine, Boston, Massachusetts
Crystal Song, DMD
Harvard School of Dental Medicine, Boston, Massachusetts
Rebekah Lucier Pryles, DMD, Certificate in Endodontics
Assistant Clinical Professor, Department of Endodontics, Tufts University School of Dental Medicine, Boston, Massachusetts; Lecturer, Department of Restorative Dentistry and Biomaterials Science, Harvard School of Dental Medicine, Boston,Massachusetts; Private Practice limited to Endodontics, White River Junction, Vermont
Brooke Blicher, DMD, Certificate in Endodontics
Assistant Clinical Professor, Department of Endodontics, Tufts University School of Dental Medicine, Boston, Massachusetts; Lecturer, Department of Restorative Dentistry and Biomaterials Science, Harvard School of Dental Medicine, Boston,Massachusetts; Private Practice limited to Endodontics, White River Junction, Vermont
References
1. AAE position statement on vital pulp therapy. J Endod. 2021;47(9):1340-1344.
2. Shabahang S. Treatment options: apexogenesis and apexification. J Endod. 2013;39(3 suppl):S26-S29.
3. Sabeti M, Huang Y, Chung YJ, Azarpazhooh A. Prognosis of vital pulp therapy on permanent dentition: a systematic review and meta-analysis of randomized controlled trials. J Endod. 2021;47(11):1683-1695.
4. Kishen A. Mechanisms and risk factors for fracture predilection in endodontically treated teeth. Endod Top. 2006;13(1):57-83.
5. Chen E, Abbott PV. Dental pulp testing: a review. Int J Dent. 2009;2009:365785.
6. Ricucci D, Loghin S, Siqueira JF Jr. Correlation between clinical and histologic pulp diagnoses. J Endod. 2014;40(12):1932-1939.
7. Virdi RS. Seltzer and Bender's dental pulp, second edition. Br Dent J. 2012;213(3):141.
8. Rechenberg DK, Zehnder M. Call for a review of diagnostic nomenclature and terminology used in endodontics. Int Endod J. 2020;53(10):1315-1317.
9. Parinyaprom N, Nirunsittirat A, Chuveera P, et al. Outcomes of direct pulp capping by using either ProRoot mineral trioxide aggregate or Biodentine in permanent teeth with carious pulp exposure in 6- to 18-year-old patients: a randomized controlled trial. J Endod. 2018;44(3):341-348.
10. Salehrabi R, Rotstein I. Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study. J Endod. 2004;30(12):846-850.
11. Reader AW. Successful local anesthesia: what endodontists need to know. American Association of Endodontists website. January 24, 2017. https://www.aae.org/specialty/successful-local-anesthesia-what-endodontists-need-to-know/. Accessed April 29, 2024.
12. Ahmad IA. Rubber dam usage for endodontic treatment: a review. Int Endod J. 2009;42(11):963-972.
13. Duncan HF, Tomson PL, Simon S, Bjørndal L. Endodontic position statements in deep caries management highlight need for clarification and consensus for patient benefit. Int Endod J. 2021;54(11):2145-2149.
14. Aguilar P, Linsuwanont P. Vital pulp therapy in vital permanent teeth with cariously exposed pulp: a systematic review. J Endod. 2011;37(5):581-587.
15. Witherspoon DE, Small JC, Harris GZ. Mineral trioxide aggregate pulpotomies: a case series outcomes assessment. J Am Dent Assoc. 2006;137(5):610-618.
16. Dhar V, Pilcher L, Fontana M, et al. Evidence-based clinical practice guideline on restorative treatments for caries lesions: a report from the American Dental Association. J Am Dent Assoc. 2023;154(7):551-566.e51.
17. Selvendran KE, Ahamed AS, Krishnamurthy M, et al. Comparison of three different materials used for indirect pulp capping in permanent molars: an in vivo study. J Conserv Dent. 2022;25(1):68-71.
18. Ballal NV, Duncan HF, Wiedemeier DB, et al. 4-year pulp survival in a randomized trial on direct pulp capping. J Endod. 2024;50(1):4-9.
19. Taha NA, Abdulkhader SZ. Full pulpotomy with biodentine in symptomatic young permanent teeth with carious exposure. J Endod. 2018;44(6):932-937.
20. Nassar H, Al-Dabbagh N, Aldabbagh R, et al. Dental follow-up and maintenance index: the development of a novel multidisciplinary protocol. Heliyon. 2020;6(5):e03954.